![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
16 Cards in this Set
- Front
- Back
|
Decrease absorption:
Ipecac |
-Consists of cephaeline (stimulates vomiting centre) and emetine (activates sensory receptors in the SI) -Limited use, short time frame -Not good for substances that can cause more injury while throwing up, e.g. caustic stuff -Affects absorption of other antidotes |
|
|
Decrease absorption:
Activated charcoal |
-Drugs adsorb onto the charcoal, so prevents absorption -May create conc. gradient across mesenteric vasculature so drug may be eliminated faster -Useful for drugs that: go through enterohepatic recirculation have small Vd low protein binding -Dose: 1-2g/kg orally or through naso-gastric tube |
|
|
Iron Poisoning |
-Ingestion of lots of iron overwhelms GI regulation and results in massive Fe absorption -When serum iron levels>capacity of binding protein (transferrin), toxicity can occur Deposition of iron in soft tissue -Free iron directly injures intestinal mucosa and generates free O2 radicals |
|
|
Neutralise the chemical:
Iron and Deferoxamine |
-Deferoxamine mesylate is an antidote for iron poisoning -Deferoxamine chelates free Fe to form feroxamine -Doesn't remove it from proteins (e.g. transferrin, ferritin, haemoglobin, cytochromes) -Feroxamine excreted unchanged in urine |
|
|
Paracetamol Poisoning |
-Paracetamol metabolises to a protein-reactive quinoneimine that reacts with sulfhydryl groups in proteins -High doses: liver failure
|
|
|
Neutralise the chemical:
Paracetamol and N-Acetyl Cysteine |
-N-Acetyl Cysteine is an antidote for paracetamol overdose -NAC is a precursor for glutathione synthesis and so elevates levels, preventing liver damage - SH group of NAC may bind and detoxify metabolite directly -NAC may act as antioxidant and blocks reactive oxygen species-dependent cell death -Loading dose + continuous infusion |
|
|
Salicylate (Aspirin) Poisoning |
-Aspirin readily hydrolysed to salicylate -Stimulates medullary respiratory center, producing hyperventilation, respiratory alkalosis and eventually metabolic acidosis -Uncouples oxidative phsophorylation increasing gluconeogenesis and lipid metabolism -Produces tinnitus, nausea/vomiting, ataxia, coma, hyperthermia >300mg/kg = serious toxic reactions >500mg/kg = potentially fatal |
|
|
Enhance Elimination:
Salicylate and Urinary Alkalisation |
-NaH2CO3 used to raise urinary pH >7.5 -Weak acids (pKa<7) that undergo significant urinary excretion become trapped in kidney tubules -Used for salicylate, phenobarbital, chlorpropamide -Monitoring needed: may produce too high pH, impairing cardiac contractility plus hypernatremia and fluid overload may occur -Used with activated charcoal |
|
|
Heroin Poisoning |
-Heroin = diacetylmorphine -Overdoses frequent when used with alcohol, cannabis, amphetamine, etc. -Many routes of administration -Narcotic effects due to conversion to morphine -Use associated with coma, seizures, and delayed encephalopathy -Lethal dose hard to define |
|
|
Pharmacodynamic Intervention:
Heroin and Naloxone |
-Naloxone is an antagonist at the μ, κ, and δ opioid receptors -Vein damage in patients? Administration by other routes -Shorter half-life than heroin, so relapse may occur -May also cause heroin withdrawal |
|
|
Warfarin Poisoning |
-Warfarin used in the prevention and treatment of venous thrombosis and pulmonary embolism -Inhibits synthesis of Vitamin K dependent coagulation factors Vitamin K reductase; Vitamin K epoxide reductase -Results in sequential depression of Factors 2, 7, 9 and 10 activities -Overdose: lots of blood/in places it shouldn't be; necrosis/gangrene; death -Also used as rodenticide |
|
|
Vitamin K Cycle |
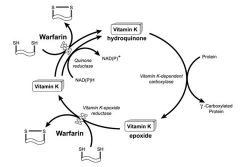
|
|
|
Replace Activity:
Warfarin and Vitamin K |
-Supplementation by Vit K needed to reverse effect -Initial dose 25-100mg/day p.o. -At higher conc, vit. K reduced to the hydroquinone by another warfarin-insensitive liver reductase -Still no cycling because Vitamin K epoxide reductase is still inhibited -Normal body stored depleted within 2-3 hours -Vitamin K therapy may be required for weeks or months until prothrombin time (coagulation) returns to normal |
|
|
Organophosphates |
-Used in agriculture and industry, and sometimes terrorism (sarin gas) -Toxicity varies -Mechanism: Inhibits acetylcholinesterase and causes a buildup of ACh |
|
|
Organophosphate Effects |
CNS Confusion Seizures
Nicotinic Effects Weakness Fasciculations Areflexia Paralysis Hypertension, tachycardia
Muscarinic Effects Increased SM contraction, gland secretions Salivation Lacrimation Urination Defecation GI Upset Pulmanory Edema |
|
|
Regenerate Target:
Organophosphates and Pralidoxime |
-Initially give atropine to antagonise ACh then pralidoxime -Pralidoxime removes phosphate group from cholinesterase enzyme to regenerate catalytic activity Effects at nicotinic>muscarinic Effect is great at erythrocytic esterase if given soon Reactivation of plasma cholinesterase activity is minimal -Most effective when give <24hrs |

