![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
56 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
what are the protein structures? |
Protein Structure Primary, secondary, tertiary and quaternary structure |
|
|
|
describe the primary structure |
Primary structure: determined by the αα sequence in the polypeptide chain. |
|
|
|
describe the secondary structure |
Secondary structure: determined by the interaction of ‘+’ charged H+ with ‘-’ charged oxygen atoms on carbons from the same polypeptide chain. e.g. α helix & β ‘pleated’ sheet. |
|
|
|
describe the tertiary structure |
Tertiary structure: is determined by the interactions of amino acids that are relatively far apart on the protein backbone. Interactions, include ionic bonds and covalent disulfide linkages (among others). |
|
|
|
describe the quaternary structure |
Quaternary structure: determined by the binding interactions among two or more independent protein subunits. |
|
|
|
explain the mechanism of the hydrophobic parts of a protein. * |
Hydrophobic parts of a protein are often drawn to the inside of a protein or protected by the lipid bi-layer. |
|
|
|
explain the mechanism of the hydrophilic parts of a protein. |
Hydrophilic parts of a protein are often drawn to the outside of a protein. The binding site is the site of the receptor at which a drug binds. The favorability of a drug for its receptor is affinity. |
|
|
|
explain Van der Waals forces |
Van der Waals forces result from polarity in a molecular induced by shifting its electron density in response to close proximity of another molecule. e.g. Hydrogen bonds |
|
|
|
explain Covalent Bonds |
Covalent bonds result from sharing a pair of electrons between two atoms.RARELY is drug binding caused by a single type of interaction.The molecular structure dictates the physical and chemical properties that contribute to specific binding. Other important factors include: hydrophobicity, ionization state pKa, confirmation & and stereochemistry. |
|
|
|
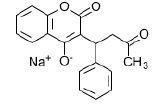
give an example of a stereoisomer resulting from covalent bonding * |
Warfarin: enantiomer is one of two stereoisomers that are mirror images of each other that are non-superposable (not identical), much as one's left and right |
|
|
|
Case 1: BCR-Abl tyrosine kinase & imatinib p3 |
.......................................................... |
..........................................................
|
|
|
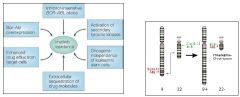
what is Imatinib (INN)? * |
Imatinib (INN): a. Gleevec (Canada, South Africa and the USA)b. Glivec (Australia, Europe and Latin America)aka STI-571, A tyrosine-kinase inhibitor used in the treatment of multiplecancers, most notably Philadelphia chromosome-positive (Ph+) chronic myelogenous leukemia (CML). |
|
|
|
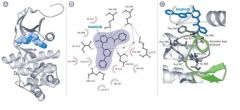
describe the Structural Basis of Specific Enzyme Inhibition * |
A. BCR-Abl tyrosine kinase. An analogue of imatinib, a specific inhibitor of the BCR-Abl tyrosine kinase, is shown.
B. Interactions between the drug and amino acid (αα) residues in the BCR-Abl protein. Hydrogen bonds (dashed lines), while van der Waals interactions (halos) are shown. C. Interaction of the drug (blue) with the BCR-Abl protein (gray) inhibits phosphorylation of the activation loop (green-ribbon). |
|
|
|
Some Pharmacodynamics: |
|
........................................................ |
|
|
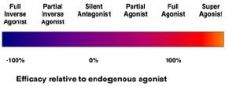
Agonists to and activate their targets causing what? |
Agonists to and activate their targets causing a change in activity. |
|
|
|
what is Super agonist capable of? |
Super agonist is capable of activating target to greater than the maximum extent. |
|
|
|
what does Full agonists bind to and activate?
|
Full agonists bind to and activate their targets to the maximal extent possible. E.g. isoproterenol, which mimics the action of adrenaline at β adrenoreceptors. |
* |
|
|
partial agonists produce what? |
Partial Agonists produce a submaximal response in binding tot their targets. E.g. buprenorphine are used to treat opiate dependence. |
|
|
|
Inverse agonists cause what? |
Inverse agonists cause constitutively active targets to become inactive. cannabinoid inverse agonist rimonabant. |
|
|
|
Irreversible agonist is what type of agonist? |
Irreversible agonist is a type of agonist that binds permanently to a receptor through the formation of covalent bonds. in such a manner that the receptor is permanently activate |
|
|
|
Antagonist inhibitor has the amility to do what? |
Antagonist inhibitor the ability of the target to be activated (or inactivated by physiologic (native) or pharmacologic (exogenous) agonists. |
|
|
|
Competitive antagonists do what? |
Competitive antagonists: drugs that directly block physiologic agonists |
* |
|
|
Uncompetitive antagonists do what? |
Uncompetitive antagonists, those that don’t compete for binding of the agonist, but affect binding by leading to conformational change in the target. |
|
|
|
4-Major Types of Drug-Receptor Interactions |
........................... |
...................................... |
|
|
type a drugs bind to? |
A. Drugs can bind ion channels, causing an alteration in conductance. |
|
|
|
type b drugs bind to? |
B. Heptahelical/7-transmembrane receptors coupled to intracellular G proteins. |
|
|
|
type c drugs bind to? |
C. Drugs can bind to the extracellular domain of a transmembrane receptor and cause a change in signaling within the cell by activating or inhibiting an enzymatic intracellular domain of the same receptor molecule. |
|
|
|
type d drugs bind to? |
D. Drugs can diffuse through the plasma membrane and bind to cytoplasmic or nuclear receptors. This is often the pathway used by lipophilic drugs (e.g., drugs that bind to steroid hormone receptors). Alternatively, drugs can inhibit enzymes in the extracellular space without the need to cross the plasma membrane (not shown). |
|
|
|
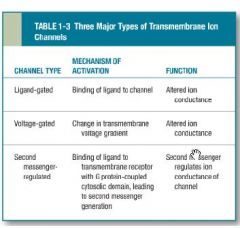
Two important classes of drugs that act by altering the conductance of ion channels are what?
* |
Two important classes of drugs that act by altering the conductance of ion channels: (i)local anaesthetics & the (ii)benzodiazepines. |
|
|
|
Local anaesthetics does what?* |
Local anaesthetics, block (sodium) Na+ channel signalling in neurons that transmit pain signals form the PNS to the CNS. |
|
|
|
how does
Benzodiazapenes act? * |
Benzodiazapenes act by inhibiting neurotransmission in the CNS by helping the gamma-aminobutyric acid (GABA) to increase the conductance of Chloride ions. |
|
|
|
Ligand-Gated Nicotinic Acetylcholine Receptor |
................................................. |
................................................ |
|
|
A. The plasma membrane acetylcholine (ACh) receptor is composed of what? |
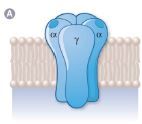
A. The plasma membrane acetylcholine (ACh) receptor is composed of: two α subunits, a β subunit, as well as a γ & δ subunits. |
|
|
|
in the absence of plasma membrane acetylcholine (ACh) receptor what happens? |
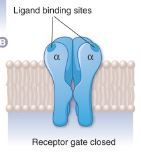
B. The γ subunit has been removed to show an internal schematic view of the receptor. In the absence of ACh, the ‘gate’ is closed. |
|
|
|
what happens When ACh is bound to both α subunits? |
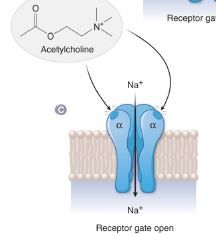
C. When ACh is bound to both α subunits, the channel opens, and Na+ can pass down its concentration gradient into the cell. |
|
|
|
table for Major G- Proteins and example of their actions. |
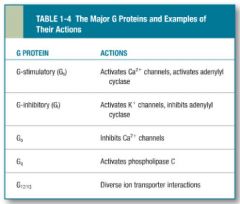
|
|
|
|
how does Receptor-Mediated G Protein Activate? |
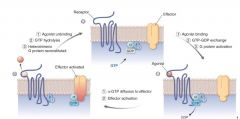
|
|
|
|
Transmembrane Receptors with Enzymatic Cytosolic Domains |
................................................ |
................................................. |
|
|
how does Receptor tyrosine kinases work? |
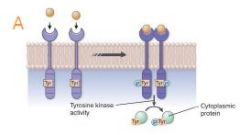
Receptor tyrosine kinases Upon ligand-induced activation, these receptors dimerize and transphosphorylate tyrosine residues in the receptor and, often, on target cytosolic proteins. |
|
|
|
how does Tyrosine phosphatases work?
|
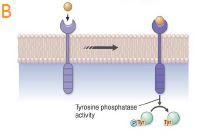
Tyrosine phosphatases. These receptors dephosphorylate tyrosine residues either on other transmembrane receptors or on cytosolic proteins. Many cells of the immune system have receptor tyrosine phosphatases. |
|
|
|
how does Nonreceptor tyrosine kinases work? |
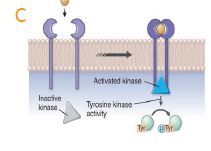
C.Nonreceptor tyrosine kinases. Some tyrosine kinase-associated receptors lack a definitive enzymatic domain, but binding of ligand to the receptor triggers activation of receptor-associated protein kinases that then phosphorylate tyrosine residues on certain cytosolic proteins. |
|
|
|
how does Receptor serine/threonine kinases work? |
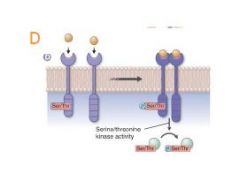
D. Receptor serine/threonine kinases phosphorylate serine and threonine residues on certain target cytosolic proteins. Members of the TGF-β superfamily of receptors are in this category. |
|
|
|
how does Receptor guanylyl cyclases work? |
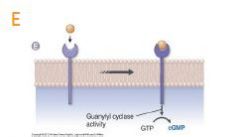
E. Receptor guanylyl cyclases contain a cytosolic domain that catalyzes the formation of cGMP from GTP. The receptor for B-type natriuretic peptide is one of the receptor guanylyl cyclases that has been well characterized. |
|
|
|
Lipophilic Molecule Binding to an Intracellular Transcription Factor/Receptor. |
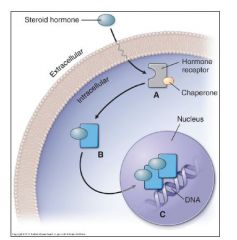
e.g. Steroid hormones |
|
|
|
Signaling Convergence of Two Receptors |
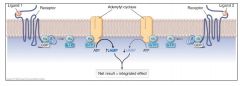
|
|
|
|
Cellular Regulation of Signaling |
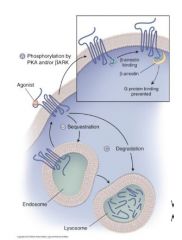
|
...................................................... |
|
|
what does Repeated/persistent stimulation of the receptor by agonist results in? |
A.Repeated/persistent stimulation of the receptor by agonist results in phosphorylation of amino acids at the C-terminus of the receptor by protein kinase A (PKA) and/or β-adrenergic receptor kinase (βARK). β-Arrestin blocks Gs binding, thereby decreasing adenylyl cyclase (effector) activity. |
|
|
|
what does Binding of β then -arrestin also lead to? |
B. Binding of β then -arrestin also leads to receptor sequestration into endosomal compartments, effectively neutralizing β-adrenergic signaling. |
|
|
|
what can Prolonged receptor occupation by an agonist lead to? |
C. Prolonged receptor occupation by an agonist can lead to receptor down-regulation & degradation. |
|
|
|
how can Cells reduce the number of receptors? |
Cells can also reduce the number of receptors by inhibiting transcription/translation.. |
|
|
|
.
|
. |
|
|
|
. |
. |
|
|
|
. |
. |
|
|
|
. |
. |
|
|
|
. |
. |
|
|
|
. |
. |
|

