![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
399 Cards in this Set
- Front
- Back
|
This is the TDLU. it is embedded in loose, specialized, hormonally responsive connective tissue stroma - intralobular stroma and the tissue between each lobule is interlobular stroma
|
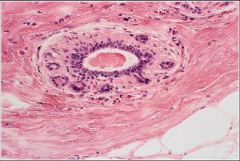
This functional unit of the breast is embedded in what tissues
|
|
|
Which lobular stromal tissue is responsive to hormones?
|
Intralobular stroma
(interlobular is not responsive to hormones) |
|
|
What is the name for the dilitation that opens up to the nipple?
|
lactiferous sinus
|
|
|
Where does lymphatic drainage from the breast go to?
|
axillary, supraclavicular and mediastinal lymph nodes
|
|
|
This is the myoepithelial cell layer whose function is to assist in expelling milk
|
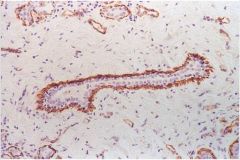
What is the function of this aspect of the ductal system of the breast?
|
|
|
Describe the progession of cell classification as you move fromt he larger ducts of the breast through the lactiferous sinus to the skin?
|
columnar epithelium at the duct to a squamous epithelial lining just distal to the lactiferous sinus then stratified squamous as it merges with the skin
|
|
|
What is breast hitology lacking in childhood?
|
lacks lobular units (has the branching ductal system)
|
|
|
What prompts the proliferation of glandualr tissue in the female breast during puberty?
|
estrogen and progesterone
|
|
|
Which parts of the breasts change with fluctuating hormone levels during the month? Which do not?
|
Changing with hormones: TDLUs
Unchanging: interlobular duct system and lactiferous ducts |
|
|
At which phase in the menstrual cycle is the intra and interlobular stroma indistinct? Highly distinct?
|
Indistinct: Follicular phase
Distinct: luteal phase |
|
|
Pregnancy and lactating breast: marked increased in # of terminal ducts and acini; TDLUs are enlarged; interlobular stroma is unchanged
|
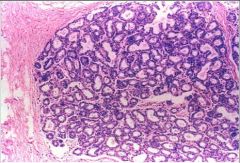
Describe the histology here. What physiological change has occured in the female?
|
|
|
This is the breast of a postmenopausal woman. TDLUs atrophy (low hormone); interlobular stroma is reduced in amount and there is an increase in amount of fatty tissue
|
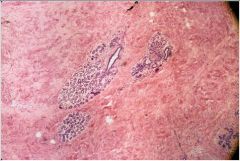
Describe the histology here. What has occurred in the physiology of the female.
|
|
|
Where may one find accessory breasts or nipples?
|
Anywhere along the mammary line travelling from the axilla to the pelvis
|
|
|
What must you ask when seeing a case of inverted nipples?
|
Is this a change in structure or have they always been inverted?
A change to inversion may be a sign of underlying cancer |
|
|
What is the pathophys of juvenile hypertrophy?
|
hormone receptors are overreacting to the change in hormone levels
|
|
|
What are the prediposing factors to gynecomastia?
|
hormonal embalance; exogenous hormones; drugs (dilatin, dig, marijuana); Klinefelter's; testicular tumors; liver disease
|
|
|
What are the THREE types of inflammatory breast lesions?
|
1. Acute mastitis and breast abcess
2. Chronic mastitis 3. Fat necrosis |
|
|
What is the most common infecting agent in acute mastitis?
|
Staph aureus
|
|
|
What should be in your Ddx for acute mastitis?
|
inflammatory breast cancer
|
|
|
Plasma cell mastitis and granulomatous mastitis closely mimic what other breast pathology?
|
breast carcinoma: irregular painless masses
|
|
|
Who are most susceptible to sub-areolar abcesses?
|
smokers
|
|
|
What are the 4 benign neoplasms of the breast?
|
1. fibroadenoma
2. lactating adenoma 3. intraductal papiloma 4. Phyllodes tumor |
|
|
What is the most common benign neoplasm of the breast?
|
fibroadenoma
|
|
|
Descibe a fibroadenoma? What is the treatment?
|
smooth, round, well-circomscribed, movable, painless
tx: excision |
|
|
When is there a risk for breast CA in a lactating adenoma?
|
only if atypia in the lining
|
|
|
At what size does a breast mass become palpable?
|
if >or= 2cm
|
|
|
What will be the typical presentation of an intraductal papilloma?
|
a single solitary mass in the area of the nipple. Will often present with bloody nipple discharge (as does CA)
|
|
|
Describe a intraducal papilloma, histologically?
|
finger-like papillae covered by a layer of epithelial and myoepithelial cells
|
|
|
Phyllodes tumors have a spectrum of agressiveness from benign to mailgnant. Where is the most common site of mets from a phyllodes tumor?
|
Lungs
|
|
|
State the number of mitoses/HPF for low, medium and high grade phyllodes tumors?
|
Low: < 5
Med: 5-10 High: > 10 |
|
|
What percentage of phyloodes tumors recurr after 2 yrs?
|
30%
|
|
|
What is the age range for dx of fibrocystic change?
|
20-55; decreases progressively after menopause
|
|
|
The cysts which develop in FCC arise from what structure in the breast?
|
TDLU
|
|
|
A benign cyst will have what color on FNA?
|
clear
|
|
|
Embryologically, breasts arise from the same lineage as what other strucutre?
|
apocrine glands
|
|
|
What is the differential dignosis in sclerosing adenosis?
|
invasive carcinoma
|
|
|
What is seen on mammography in sclerosing adenosis that can be mistaken for CA?
|
diffuse micorcalcifications
|
|
|
What will you see microscopically in sclerosing adenosis?
|
proliferation of acinar structures and stroma, dostorting the TDLU; acini produced whorls and cords
|
|
|
FCC can be proliferative or non-proliferative. Which features are distinct to proliferative vs. nonproliferative FCC?
|
Non-proliferative: (+) cycts and fibrosis, (-) epithelial hyperplasia
Proliferative: (+) cysts/fibrosis AND (+) lobular and ductal epithelial hyperplasia |
|
|
What distinguishes Atypical lobular hyperplasia (ALH) from Lobular carcinoma in situ (LCIS)?
|
ALH: < 50% of lobules are filled with epithelial cell proliferation
LCIS: > 50% are filled and distented by epithelial proliferation |
|
|
What are the 3 forms of ductal hyperplasia?
|
1. usual type
2. atypical ductal hyperplasia 3. ductal carcinoma in situ |
|
|
What defines ductal carcinoma in situ?
|
malignant cells are confined within basement membranes of ducts -- no invasion of surrounding stroma
|
|
|
Which benign and pre-malignant epithelial proliferation gives NO INCREASED RISK of breast CA?
|
cyst, apocrine metaplasia, sclerosing adenosis, fibrosis and mild hyperplasia
|
|
|
Which benign and pre-malignant epithelial proliferation gives SLIGHTLY INCREASED RISK (1.5-2x) of breast CA?
|
moderate or florid hyperplasia
papilloma |
|
|
Which benign and pre-malignant epithelial proliferation gives MODERATELY INCREASED RISK (4x) of breast CA?
|
ADH
ALH |
|
|
Which benign and pre-malignant epithelial proliferation gives MARKEDLY INCREASED RISK (10x) of breast CA?
|
DCIS
LCIS |
|
|
Which carcinoma in situ (DCIS or LCIS) is a marker for increased risk of developing invasive carcinoma in either breast and either ductal or lobular?
|
LCIS
|
|
|
What is the most common malignant tumor of the breast?
|
carcinoma
|
|
|
What percentage of breast CA is inherited?
|
5-10%
|
|
|
BRCA1 and BRCA2 are genes that function is what capacity?
|
DNA repair
|
|
|
Which genetic mutation is found in 50% of familial cases of Breast CA?
|
BRCA1
|
|
|
What is the location of BRCA1?
|
17q21
|
|
|
What is the location of BRCA2?
|
13q12-13
|
|
|
Which gene is amplified in 30% of BrCA pts?
|
HER2
|
|
|
What other genes (aside from HER2)can also be amplified in BrCA?
|
RAS, MYC
|
|
|
Which tumor suppressor genes may be mutated in BrCA?
|
RB1 (retinoblastoma) and P53
|
|
|
Where is the site of origin for breast CA?
|
TDLU
|
|
|
What are the four main types of Ductal CIS and which is high grade?
|
Comedo (high grade; central necrosis)
Cribiform (punched out holes) Micropapillary Solid |
|
|
High grades of DCIS often express which gene?
|
HER2neu
|
|
|
What fraction of DCIS develop invasive carcinoma?
|
1/3; usually same breast and same quadrant
|
|
|
What is the Tx DCIS?
|
surgery and radiation; tamoxifen to decrease risk of recurrence of DCIS and subsequent invasive carcinoma
|
|
|
What is the Tx for LCIS?
|
negative margins are not a necessity in LCIS; tamoxifen reduces recurrence risk and development of carcinoma
|
|
|
what is the hiological pattern of Invasive lobular carcinoma?
|
single cells invading stroma in "indian file pattern" (one nucleus after another in a line)
|
|
|
Which type of carcinoma accounts for 70-80% breast carcinoma?
|
Invasive ductal carcinoma - no special type
|
|
|
What is a good prognositc sign in Invasive ductal carcinoma -NST? Poor px?
|
ER/PR + = good
Her 2 neu + = poor |
|
|
Invasive lobular carcinoma is frequently associated with which precursor pathology?
|
LCIS
|
|
|
Where do invasive lobular carcinomas frequently mets to?
|
CSF, serosal surfaces, uterus, ovaries, bone marrow
|
|
|
Which form of invasive carcinoma is more likely to multifocal and bilateral?
|
Invasive lobular carcinoma
|
|
|
What is the usual genetic markers of invasive lobular carcinoma?
|
ER/PR + and Her 2 neu -
|
|
|
What does it mean that meduallry carcinomas have a triple negative profile?
|
ER/PR -, Her 2 neu -
|
|
|
Which form of breast carcinoma has an extremely poor px due to tumor invasion into lymphatic spaces in the breast and skin?
|
Inflammatory carcinoma (does not cause inflammation)
|
|
|
What are the three criteria used to give a histologic grade to breast cancer?
|
amount of tubule formation (1-3 pt)
number of mitoses (1-3 points) degree of nuclear atypia (1-3 points) |
|
|
Which immunohistochemistry test is useful in distinguishing DCIS from LCIS?
|
E-cadherin: present in DCIS
|
|
|
Why does an ER/PR + finding indicate a better prognosis for breast carcinoma?
|
the tumor may respond to Tamoxifen
|
|
|
What is the importance of the Her2/neu status in treatment for breast CA?
|
Her2/neu status is a predictor of response or resistance to various forms of systemic therapy (anthracycline vs. CMF)
|
|
|
What monoclonal antibody to Her2/neu protein may prolong survival of pts with metastatic dz?
|
Herceptin
|
|
|
A FISH score great than ___ is considered positive for gene amplification of Her 2
|
3
|
|
|
What are the three criteria used to stage breast cancer (thus conferring prognosis)
|
1. tumor size
2. lymph node involvement 3. presence or abscence of distant mets |
|
|
Of the three staging criteria, which is the most important prognostic indicator?
|
Lymph node involvement
|
|
|
What is Paget's disease?
|
extension of DCIS up the lactiferous duct to involve the overlying epidermis of the nipple
|
|
|
What percentage of Paget's dz has underlying invasive cancer?
|
50%
|
|
|
How does Paget's dz tyically present?
|
Crusting of the nipple
|
|
|
What two layers of cells surround the glands in normal breast tissue?
|
Epithelial layer and Myoepitheal layer
|
|
|
What is the role of PTHrP in breast development? What happens in its absence?
|
induces differentiation from dermal mesenchyme to mammary mesenchyme; amastia = Bloomstrands chondroplasia
|
|
|
What contributes to breast development during puberty?
|
fatty tissue increase, not glandular development
|
|
|
What happens to the tdlu of the breastduring the luteal phase of the menstrual cycle?
|
progesterone induces side-branching and lobule-alveolar development
|
|
|
What accounts for the branching and lobule formation in breast tissue sometimes seen in infancy?
|
PRL enters the placental blood
|
|
|
How is primary ductal development regulated during puberty?
|
Estrogen, IGF-1 and GH
|
|
|
What changes occur in the breast during pregnancy?
|
alveolar proliferation and maturation; milk protien expression initiated but secretion inhibited by high progesterone
|
|
|
What two things encourage milk secretion during lactation?
|
Prolactin levels (which increase with suckling due to pulsatile DA inhibition) and milk removal
|
|
|
What two things encourage milk ejection during lactation?
|
suckling and oxytocin
|
|
|
How does obesity affect lactation?
|
negative impact due to inadequate PRL release
|
|
|
What differences exist between milk in humans vs. other animals?
|
higher carbohydrate levels, different mineral concentrations, human is IgA positive
|
|
|
how does oxytocin affect milk secretion?
|
causes myoepithelial cell contraction
|
|
|
How is IgA secreted in breask milk?
|
tight junctions remain open briefly after parturition
|
|
|
What factors that influence the neuroendocrine reflex can affect lactation?
|
anxiety/stress, pituitary disorders, obesity
|
|
|
What is the classification of the human placenta?
|
Chorioallantoic (vasculature), discoid (shape), villous, hemorchorial
|
|
|
Which cells of the placenta are in direct contact with maternal blood?
|
trophoblast cells (syncytiotrophoblasts)
|
|
|
Describe primary villi of the placenta?
|
cytotrophoblast core surrounded by syncytiotrophoblast
|
|
|
Describe secondary villous of the placenta?
|
extraembryonic mesoderm forming a villous core
|
|
|
Tertiary villi of the placenta are characterized by what?
|
formation of the arteriocapillary network
|
|
|
When in embryologic development does the villous stage occur?
|
Days 13-18 after conception
|
|
|
What is normally seen on an H and E stain of a Tertiary Villi in the placenta at the beginnng of pregnancy?
|
Two cell layers (inner = cytotroph, outer = syncytiotroph), stromal core with arterial formation filled with fetal blood
|
|
|
How can you tell fetal from maternal blood?
|
Fetal blood has more nucleated RBCs
|
|
|
Which villi make up the majority of the placental mass?
|
Floating villi
|
|
|
What are the 3 functions of the anchoring villi?
|
1. attachment to the uterus
2. form cell column, allowing it to invade into the decidua and myometrium 3. remodeling of the spiral arteries |
|
|
What is the process by which the anchoring villi cell walls remodel the maternal spiral arteries into fetal cell arteries?
|
apoptosis
|
|
|
What do floating villi look like at term?
|
very thin syncytial layer; no more two cell layer; sinusoids buffered right up against the syncytia to facilitate more diffusion
|
|
|
What constitutes the chorion?
|
fetal trophobasts and maternal decidua
|
|
|
What is a ghost villi?
|
a remnant structure of chorionic villi where stromal core used to be
|
|
|
Where does the entire gestation take place in embryologic development?
|
All within the entrie wall of the uterus
|
|
|
T/F: the placenta precedes the development of the embryo
|
True
|
|
|
What are hte 6 functions of the placenta?
|
1. transport
2. respiration 3. hepatic 4. skin (barrier of infection/modulate temperature) 5. endocrine 6. immune system |
|
|
What are the three mechanisms of placental transport?
|
1. diffusion (gases/water)
2. facilitated diffusion (glucose) 3. active transport (amino acids) |
|
|
What is the rate-limiting step in diffusion-limited (slow)transport?
|
the rate of movement across the syncytiotrophoblast membranes between the intervillous space and the fetal capillaries
|
|
|
What is the rate-limiting step in flow-limited (fast)transport?
|
plasma concentration and the rate of blood flow
|
|
|
What are the differences in fetal hemoglobin versus maternal hemoglobin?
|
Fetal Hb:
- different beta chains - greater affinity for O2 - lower concentration of 2,3 diphosphoglycerate |
|
|
What is one of the first peptide hormones made by the trophoblasts of the placenta?
|
hCG (peaks at 10 weeks of PG and then declines)
|
|
|
What is the function of hCG?
|
1. maintains the excretion of progesterone in the corpus luteum
2. regulates cytotrophoblast differentiation into syncytiotrophoblasts |
|
|
Where is hPL (human placental lactogen) produced?
|
Syncytiotrophoblasts in placenta
|
|
|
What is the function of hPL?
|
1. creates an insulin-resistant state in the mom, making carbs more available to the fetus
|
|
|
Which peptide hormone secreted by the placenta is involved in gestational diabetes?
|
hPL
|
|
|
What are the functions of progesterone in pregnancy?
|
suppresses uterine contractions
|
|
|
Describe the placenta - fetus relationship in the production of steroid hormones?
|
the placenta can produce progesterone; only the fetus can produce the androgens; the placenta can produce the estrogens fromthe androgens that the fetur produced
|
|
|
What are the macrophages of the placenta and where are they located?
|
Hofbauer cells in the villous core
|
|
|
What it the function of Hofbauer cells?
|
transports maternal IgG to fetal circulation (fetus can make IgM)
|
|
|
What does it mean if you find IgM against a pathogen in fetal blood?
|
The fetus has seen that pathogen
|
|
|
What is fetal hydrops?
|
transport of IgG to the Rh factor; mother develops antibodies to the Rh antigen from first child and will degrade Rh of the second fetus causing anemia and death if not treated
|
|
|
What do you give to a mother with Rh antigen to her first fetus?
|
Rhogam
|
|
|
What are the main placental estrogen deficiencies?
|
1. placental desulfate deficiency (failure to initiate labor)
2. P450 aromatase deficiency (virulization of mother and fetus) |
|
|
What is the composition of amniotic fluid?
|
fetal urine and lung secretion
|
|
|
Oligohydraminos causes what sx in the fetus?
|
pulmonary hypoplasia
skeletal muscular defects |
|
|
What are the causes of oligohydramnios?
|
1. rupture of membranes
2. congenital anomalies (GU system) 3. nephrotoxic drugs (ACE inhibitors, NSAIDS) 4. poor placental perfusion |
|
|
What are the causes polyhydraminos?
|
1. congenital anomalies (neural tube defects, esophageal atresia)
2. gestational diabetes |
|
|
The majority of twins are of what type?
|
Dizygotic
|
|
|
Dizygotic twins end up with how many placentas/waterbags?
|
2 of each
|
|
|
What determines the number of placenta and water bags that monozygotic twins have?
|
The time of the split: THE LATER YOU SPLIT, THE MORE YOU SHARE
|
|
|
70% of diamniotic/monochorionic twins occur with a split at what day range?
|
days 3-8
|
|
|
A split on what day equates to conjoined twins?
|
unlucky day 13
|
|
|
Why does sharing a waterbag equate to high fetal mortality?
|
cord entanglement
|
|
|
What are you looking for on ultrasound to know you have a dichorion placenta?
|
"twin peak" or lambda sign (vs. T of a monochorion)
|
|
|
What is Twin-twin transfusion syndrome?
|
an unequal sharing of the blood between the two pregnancies; anastamoses of vessels between twins where blood can get shunted to the other fetus
|
|
|
What is the relative mortality rate of TTTS?
|
70-90%
|
|
|
What do the twins of TTTS look like?
|
donor - oligohydraminos, smaller
receiver - polyhydraminos, large, edematous |
|
|
What is preeclampsia?
|
edema, proteinuria and htn after 30th week of pregnancy
|
|
|
What is eclampsia?
|
seizures in pregnancy
|
|
|
What structure is a key player in preeclampsia?
|
the placenta; once the placenta is delivered, the dz is completely resolved
|
|
|
How do we know that it is not the fetus that causes preeclampsia?
|
in molar pregnancies (no fetal tissue) you can still get preeclampsia
|
|
|
What is the pathophys of preeclampsia?
|
difference in the invasive ability of the morula and the remodeling of the spiral arteries by the anchoring villi (poor placentation); placenta releases toxic factor that gets into maternal circulation -> endothelial dysfunction and inflammatory response
|
|
|
What structure is a key player in preeclampsia?
|
the placenta; once the placenta is delivered, the dz is completely resolved
|
|
|
How do we know that it is not the fetus that causes preeclampsia?
|
in molar pregnancies (no fetal tissue) you can still get preeclampsia
|
|
|
What is the pathophys of preeclampsia?
|
difference in the invasive ability of the morula and the remodeling of the spiral arteries by the anchoring villi (poor placentation); placenta releases toxic factor that gets into maternal circulation -> endothelial dysfunction and inflammatory response
|
|
|
In the placenta, which vessel carries oxygenated blood?
|
Vein
|
|
|
what is the clinical significance of a bilobed placenta?
|
usually no significance
|
|
|
What is a succenturiate lobe?
|
a small segment of the placental disc attached to the main disc by small vessels; can result in post-partum hemorrhage
|
|
|
What is a circumvallate placenta?
|
the free membrane is folding along its circumfrential attachment to the placental disc
|
|
|
What is agenesis?
|
complete failure to form a structure
|
|
|
What is aplasia?
|
tiny remnant of tissue represents a failed attempt to form the structure
|
|
|
What is hypertrophy?
|
individual cells get larger leading to a larger structure (rare in neonates)
|
|
|
What is hyperplasia?
|
There are more cells in a tissue leading to a larger structure (more common in neonates)
|
|
|
What is dysplasia?
|
an abnormally formed structure; often resembles the embryonic stage of development of the affected organ
|
|
|
What are the three types of congenital dysmorphism?
|
Malformation
Deformation Disruption |
|
|
What is the difference between "present at birth" and "manifest at birth"?
|
Presenta at birth - the defect exisits at birth but may not necessarily show itself until a later time
Manifest at birth - the defect is apparent at birth |
|
|
What is malposition (ectopia)?
|
structure is present but in an abnormal location
(e.g. Transposition of great vessels) |
|
|
What is a fusion defect?
|
Structures which normally form by fusion and fail to do so (e.g. cleft palate) OR structures which are normally unconnected may fuse or fail to separate (e.g., horseshoe kidney)
|
|
|
What is atresia?
|
when a nornally tubular structure has no lumen
|
|
|
What is stenosis?
|
When a normally tubular structure has a narrowed lumen
|
|
|
What is a deformation? When do they occur?
|
abnormality in the form, shape or position of a structure which is due to an external mechanical factor compressing the structure; occurs before birth during or after the formation of the structure
|
|
|
What is disruption?
|
a defect in a structure which is due to an external mechanical factor which "disrupts" the developing of already developed structure in utero.
|
|
|
What is an amniotic band? What form of dysmorphism is an amniotic band?
|
tears int he placental membrane covering the fetal surface of the placental disc; tear forms a band which may attach to or wrap around a fetal structure or the umbilical cord; it is an example of disruption
|
|
|
What is a distinguishing characteristic between deformations/distruptions and malformations?
|
deformations/disruptions are usually spontaneous and unlikely to recur in future PG's; malformations more likely to have genetic syndromes associated with it
|
|
|
What should you look for in a neonate with multiple malformations?
|
hereditary disorders, karyotpic abnormality or teratogens
|
|
|
What is a syndrome?
|
a characteristic patern of dysmorphisms
|
|
|
What are the characteristics of Meckel's Syndrome?
|
stillborn with polydactyly, cephalocele, and bilateral renal dysplasia
|
|
|
What are the characteristics of Holt-Oram syndrome?
|
newborn with upper limb deformity and atrial septal defects
|
|
|
What defines a VATER Association?
|
Vertebral/Anal/Tracheal-Esophageal fistula with Radial or Renal malformations
|
|
|
What is the term used when one or more congenital dysmorphism leads to other dysmorphism?
|
Sequence
|
|
|
What is the clinical triad of chromosomal disorders?
|
1. abnormal growth
2. dysmorphisms 3. CNS dysfunction |
|
|
What is the function of transcription factors?
|
interact with DNA to affect the expression of other genes
|
|
|
What are the three main developmental genes that are expressed via transcription factors?
|
1. growth factors
2. cell surface proteins 3. receptors |
|
|
Inhibition of Sonic Hedgehog (SHH) leads to defects in what developmental fields?
|
right-left body asymmetry
|
|
|
What is a phenocopy?
|
similar or identical phenotype resulting from different mechanisms
|
|
|
Tetrology of Fallot is an example of what mechanism of defect? What are the causes of Tet?
|
ToF is an example of phenocopy; genes are deleted on Ch 22q11 and Vitamin A receptors can be overstimulated by 13 cis-retinoic acid (Accutane)
|
|
|
What is genetic heterogeneity?
|
mutations in different genes that can cause the same disorder (ToF is also an example of genetic heterogeneity)
|
|
|
What does the threshold model state?
|
that a particular genotype establishes hereditary predisposition but environment will affect the threshold for maldevelopment
|
|
|
What are three examples of defects that can be isolated or syndromic?
|
1. Congenital heart disease
2. Cleft lip and cleft palate 3. Club foot |
|
|
What are the major causes of congenital malformations?
|
unknown (largest)
multifactorial chromosomal Monogenic |
|
|
What are the five hormones effecting fetal groeth and development?
|
hCG, hPL, hPGH, estrogen, progesterone
first three are polypeptide hormones |
|
|
Most of the placental hormones are made by which cells of the placenta?
|
syncytiotrophoblasts
|
|
|
Who is more effected by the placental hormones (mother or baby)?
|
Mother since the syncytiotrophoblasts are bathes in mother's blood
|
|
|
What pituitary hormones are similar to hCG?
|
LH, FSH, and TSH share alpha subunits
LH shares b-subunits |
|
|
When in gestation does hCG peak?
|
10-12 weeks
|
|
|
What are the functions of hCG?
|
1. maintains corpus luteum progesterone
2. controls trophoblastic invasiveness 3. decreases miometrial contractility in early pregnancy 4. binds to TSH receptor and has TSH activity at high levels 5. acts like LH and stimulates testosterone in leydig to differentiate internal and external genetalia |
|
|
What happens to TSH when hCG is present?
|
hCG binds to thyroid hormone and suppresses TSH; PG woman does not have Grave's
|
|
|
When does hPL peak in gestation?
|
30-34 weeks
|
|
|
What are the biological activites in hPL?
|
1. stimulates insulin secretion
2. promotes growth of mammary tissue 3. facilitates mobilization and utilization of FFA for energy |
|
|
What is the role of hPGH?
|
1. replaces pituitary GH in maternal circulation by 20 weeks
2. regulates transplacental glucose and AA transport |
|
|
What is the main insulin-resistance hormone in PG and why is this important?
|
hPGH
increases nutrient availability to the fetus |
|
|
How does the fetus protect itself from too high levels of progesterone?
|
fetus lacks 3b-hydroxysteroid dehydrogenase so it cannot synthesize progesterone from maternal cholesterol
|
|
|
From where is fetal cortisol synthesized?
|
from placental progesterone
|
|
|
What is the substrate for placental progesterone?
|
maternal circulating LDL and VLDL
|
|
|
By how much do estrogen levels increase in pregnancy?
|
100-fold beginning at 6 weeks
|
|
|
Who receives the majority of the synthesized estrogen?
|
Mother receives 90%
|
|
|
The leading cause of maternal death in PG is due to what hormone and why?
|
Estrogen; increases clotting factors and fibrinogen; PE is the leading cause of death in pregnany women
|
|
|
What hormone causes the priming of the uterus for labor?
|
estrogen; increases oxytocin receptors and induces myometrial gap jnxn formation by Cx-43
|
|
|
The placenta and fetus are required for the synthesis of one hormone. What is it?
|
Estradiol
|
|
|
When is the insulin resistant state in pregnancy?
|
mid to late PG
|
|
|
What is the REAL definition of GDM?
|
glucose intolerance recognized for the first time during PG
|
|
|
Thin women who develop GDM present with what signs?
|
antibodies to glutamic acid decarboxylase (GAD) - consistent with Type 1 diabetes
|
|
|
GDM patients have insulin resistance all the way to what organ and what is the consequence of this?
|
Insulin resistance at the liver; fasting hyperglycemia
|
|
|
What are the sequelae of GDM in mothers?
|
1. 50% chance of developing Type 2 DM in 5-10 years
2. higher risk of infections and C-section |
|
|
What are the effects of GDM or Type II DM on the baby?
|
1. increased risk of glucose intolerance, obesisty, DM as it gets older
|
|
|
What complication of PG leads to small babies in Type I DM?
|
improper placentation
|
|
|
TSH is suppressed in the 1st trimester by what hormone?
|
hCG
|
|
|
What hormone increases hepatic production of TH binding globulin?
|
estrogen
|
|
|
What needs to be measured in order to make a diagnoses of hyper/hypo thyroidism?
|
Free T4/T3 - it will stay normal in PG even though total T4/T3 goes up
|
|
|
How long is the fetus dependent on mom's thyroid hormone axis?
|
until the first 18 weeks (mom is hypothyroid)
|
|
|
What is the leading preventable cause of mental retardation?
|
iodine deficiency
|
|
|
What mineral is necessary to prevent cretinism/TH deficiency in neonates?
|
iodine
|
|
|
What is the molecule responsible for keeping the chromatin condensed in the sperm head?
|
Protamine
|
|
|
What is the function of protamine?
|
a histone in the sperm head that tightly wraps DNA via disulfide bonds
|
|
|
Oocytes arrested at prophase of Meiosis I are called...
|
Primary oocytes
|
|
|
What is the purpose of asymmetric cell division completed by oocytes after the first meiotic division?
|
it ensures that the majority of cytoplasm will go to the secondary oocyte (vs the first polar body)
|
|
|
When do oocytes resume meiosis and generate the second polar body?
|
after fertilization
|
|
|
Define fertilization
|
the process involving union of male and female germ cells that results in the formation of a pronuclear zygote
|
|
|
What factors are assessed in a semenanalysis?
|
color, volume, pH, motility, morphology, progression
|
|
|
Define capacitation
|
the process by which spermatazoa become able to undergo the acrosome rxn -> fertilize eggs
|
|
|
What are capacitated sperm able to do to the egg that immature sperm cannot?
|
bind to the zona pellucida
|
|
|
What are the 5 steps to the sperm-egg interaction?
|
1. Zona Pellucida binding
2. Acrosome rxn 3. ZP penetration 4. oolemma fusion 5. intravitelline processing |
|
|
Which glycoprotein of the zona pellucida mediated sperm binding?
|
ZP3
|
|
|
What constitutes the acrosome reaction?
|
when the outer membrane of hte acrosome region fuses with the plasma membrane of the sperm -> hyaluronidase and acrosin released -> fusion of sperm with oocyte
|
|
|
What protein is responsible for the fusion of sperm and oocyte?
|
fertilin
|
|
|
During the zona reaction, what is protein inhibits the binding of new sperm?
|
ZP3-F
|
|
|
Define polyspermy
|
more than one sperm fertilizing an egg
|
|
|
What occurs during oocyte activation?
|
the oocyte undergoes the 2nd meiotic division and produces the second polar body
|
|
|
What protein is responsible for the reduction of the disulfide bonds in the sperm chromatin and where does it come from?
|
Glutathione, oocyte-derived
|
|
|
What will the connecting stalk eventually become to the fetus?
|
umbilical cord
|
|
|
How long does the morula typically linger in the uterus before beginning implantation?
|
2-3 days
|
|
|
Describe hatching. When does it occur?
|
On day 5 the developing blastocyst escapes from the zona pallucida to prepare for attachment to the endothelium
|
|
|
What cells cover the placental villi and are therefore in direct contact with maternal blood?
|
syncytiotrophoblasts
|
|
|
What allows the radial arteries to be unresponsive to vasoactive substances of maternal blood?
|
they are lined with trophoblasts
|
|
|
When does decidualizaton occur?
|
late in the luteal phase
|
|
|
What are the 5 changes that occur in decidulization?
|
1. differentiation of stroma fibroblasts into decidual cells
2. change in the nature of hte ECM (more hyaluronan, less collagen VI) 3. prdxn of prolactin and IGFBP-1 by uterine stromal cells 4. Activation of COX2 -> increased synthesis of PGE2 5. recruitment of dNK (natural killers) to decidua |
|
|
What chemokine receptor is expressed in dNK cells?
|
CXCR4
|
|
|
What are the probably roles of dNK cells?
|
1. antiviral actions
2. assist in uterine remodeling 3. immune protection of the maternal-fetal interface |
|
|
What are syncytiotrophoblasts lacking that may otherwise provoke rejection of the fetus from the mother?
|
MHC antigens
|
|
|
The preimplantation embryo is characterized by the production of which 5 factors?
|
1. Heparin-binding EGF-like growth factor (HB-EGF)
2. MMP2 and TIMPS 3. hCG 4. IL-1a and IL-1b 5. IL receptors IL-1ra and IL-1R |
|
|
What are the 4 stages of implantation?
|
1. apposition
2. adhesion 3. penetration or engulfment 4. spiral artery invasion and dilatation |
|
|
During apposition, what structures of the uterine wall interact with the microvilli on the blastocyst surface?
|
pinapodes (formed via progesterone release)
|
|
|
What protein is upregulated on the uterine surface to promote attachment?
|
Mucin MUC-1
|
|
|
What factors are thought to be important signaling molecules for blastocyst/epithelium interaction?
|
IL-1, LIF (leukemia inhibitory factor), EGF
|
|
|
What integrins are expressed on the glandular epithelium of the uterus that carrelate with attachment of the blastocyst?
|
aVb3 and a4b1
|
|
|
What are the first blastocyst cells to penetrate the uterine epithelium in the invasion stage?
|
syncytiotrophoblasts followed by mononuclear cytotrophoblasts
|
|
|
How is gestational age calculated?
|
Week 0 starts the first day of the last menstrual period
|
|
|
How long is a normal term pregnancy?
|
37 weeks through 41 weeks, 6 days
|
|
|
What tests make up the routine antenatal labs?
|
Blood type (ABO/Rh), Hgb, rubella, HIV, RPR, Hep B, Urine Culture, CT/GC PCR, Pap smear
|
|
|
At what point in pregnancy should women be tested for Gestational DM? What is the test?
|
Early and again at 24-28 weeks; Oral glucose challenge (50g, 1 hour)
|
|
|
What is the normal BP pattern in pregnancy?
|
Decrease in 1st, early 2nd trimester, increase to pre-pregnancy levels in 3rd
|
|
|
For the following pregnant hypertensive disorder, state 1) bp cutoffs 2) time cutoff 3) urine protein status : PREECLAMPSIA
|
SPB > 140 OR DBP > 90 AFTER 20 weeks gestation, proteinuria>300mg (24 hr collection)
|
|
|
For the following pregnant hypertensive disorder, state 1) bp cutoffs 2) time cutoff 3) urine protein status : GESTATIONAL HYPERTENSION
|
SPB > 140 OR DBP > 90 AFTER 20 weeks gestation, NO proteinuria
|
|
|
For the following pregnant hypertensive disorder, state 1) bp cutoffs 2) time cutoff 3) urine protein status : CHRONIC HYPERTENSION
|
SPB > 140 OR DBP > 90 BEFORE 20 weeks gestation, NO proteinuria
|
|
|
For the following pregnant hypertensive disorder, state 1) bp cutoffs 2) time cutoff 3) urine protein status : PREECLAMPSIA superimposed on CHRONIC HYPERTENSION
|
HTN ecxacerbation, new onset / increased proteinuria, multisystem involvement (ie thrombocytopenia, transaminitis)
|
|
|
For the following pregnant hypertensive disorder, state 1) bp cutoffs 2) time cutoff 3) urine protein status : SEVERE PREECLAMPSIA
|
SPB > 160 OR DBP > 110 (2 readings 6hrs apart), proteinuria>5g in 24 hrs, also visual disturbances, epigastric pain, nausea, vomiting, pulmonary edema, impaired liver function, thrombocytopenia
|
|
|
What are the biggest risk factors for preeclampsia?
|
previous self/Family Hx, nulliparity, high BMI, HTN, age>40, DM, renal disease, autoimmune disease, twins
|
|
|
What are the three main mechanisms of preeclampsia?
|
Abnormal Placentation, Endothelial Dysfunction, increased angiogenic factors
|
|
|
What are the MATERNAL complications of preeclampsia?
|
pulmonary edema, oliguria, seizures, HELLP syndrome (Hemolysis, Elevated Liver functions, Low Platelets)
|
|
|
What are the FETAL complications of preeclampsia?
|
growth restriction, oligohydramnios, placental abruption
|
|
|
What is the BP threshold for TREATING preeclampsia?
|
> 105
|
|
|
What is the cornersone Tx for preelampsia?
|
Delivery
|
|
|
What are the MATERNAL indications for early delivery in preeclampsia?
|
GA > 38 weeks, Platelets < 100,000, placental abruption, deline in liver/renal fxn, headaches/visual changes, epigastric pain/nausea/vomiting
|
|
|
What are the FETAL indications for early delivery in preeclampsia?
|
abnormal antepartum testing, severe growth restriction, oligohydramnios,
|
|
|
When is the APGAR score performed?
|
1 and 5 minutes, then every 5 min until > 6 or out of delivery room
|
|
|
T/F: The APGAR score is an excellent predictor of long term health for the neonate.
|
FALSE: Only indicates bad long term outcome if less than 3 for >10-15 minutes
|
|
|
What drug has been shown to decrease the risk of preeclampsia in high-risk women?
|
Baby aspirin
|
|
|
If the GDM screening test is positive, what test confrims the diagnosis?
|
100g, 3 hour glucose challenge (w/3 days of carb loading) ; HbA1C
|
|
|
What is the preferred treatment for GDM?
|
Diet (carb restriction) and Exercise (30 min x 5 days/week), glucose monitoring
|
|
|
When during pregancy are GDM Sx the worst? Why?
|
3rd trimester; Rising placental hormones (lactogen, GH) antagonize insulin
|
|
|
If required, what pharmacologic therapy is used in GDM?
|
Insulin (NPH, regular), Glyburide (does not cross placenta - most other hypoglycemic agents do!)
|
|
|
Why do GDM fetuses have polyhydramnios?
|
Maternal hyperglycemia -> Fetal hyperglycemia -> Fetal polyuria (osmotic effect) -> Increased fluid in amniotic sac
|
|
|
What are the methods available for detecting breast cancer?
|
self-exam, clinical exam, mammography
|
|
|
What size lesion is detectable on clinical breast exam
|
2 cm
|
|
|
What is the difference between a screening mammogram and a diagnostic mammogram?
|
Screening: asymptomatic women to find occult malignancy; Diagnostic: management or follow-up on a pt with an abnormality
|
|
|
What information is included in a BI-RADS report?
|
1. Indication for exam 2. overall density 3. specific findings (if any) 4. comparison to previous 5. assessment/recommendations
|
|
|
What are the target goals for PPV of abnormal mammogram and prevalent Ca on 1st Mg?
|
PPV = 5-10%, prevalent ca on 1st Mg = 6-10/1000
|
|
|
With the exception of birth control pills, what do most breast cancer risk factors have in common?
|
Prolonged estrogen exposure: early menarche, late menopause, HRT, older at 1st Pg
|
|
|
What percentage of breast cancer in the U.S. is related to familial inherited BRCA genes?
|
only 2-3%
|
|
|
Who should not use the Gail model for predicting breast cancer risk? Why?
|
Fam hx of 3 or more cases, Ashkenazi Jewish with at least 1 case, a woman with previous or current case(case = either ovarian or breast). Gail model with UNDERestimate their risk.
|
|
|
What questions does the Gail model for breast cancer risk ask (7 total)?
|
Age, previous DCIS or LCIS, number of 1st degree relatives with Br Ca, age at menarche, age at first delivery, race/ethnicity, previous breast biopsy
|
|
|
At what age should women start having regular mammograms?
|
40
|
|
|
In addition to mammograms for women 40+, what recommendations does the ACS provide?
|
CBE q3 yrs for women 20-39, SBE starting at age 20, CBE in additon to mammography for women> 40
|
|
|
In determining whether a pt should be tested for the BRCA germline mutations, what are determinants of risk?
|
Jewish descent, number, gender and relationship of affected relatives, age at family member dx, type of Ca in relative
|
|
|
What two results does the Gail model for breast cancer risk provide?
|
5 yr risk and lifetime risk
|
|
|
Why is the lifetime risk of breast cancer always so much higher?
|
because a woman's risk of breast cancer increases with age
|
|
|
If your 5yr risk is high according to the Gail model, what will your relative lifetime risk be?
|
it will remain higher than average
|
|
|
What procedures can be performed to determine whether a palpable breast lump is malignant?
|
fine needle aspiration - single cells with cytologic atypia & intact cytoplasm, or mass biopsy
|
|
|
What technique yields the thickest core breast biopsy?
|
Mammotome
|
|
|
What surgical options are available to excise a malignant breast mass? What are the indications for each?
|
lumpectomy - palpable mass, needle localization - seen on mammogram only, radical option -modified mastectomy
|
|
|
What information should be included on pathology report of breast carcinoma?
|
histologic type, in situ vs invasive, size of tumor, margins + or - for carcinoma, angiolymphatic invasion if present
|
|
|
What two histologic types of breast cancer predominate?
|
ductal tries to form glands, lobular - single cells in Single File (Indian File)
|
|
|
Size of tumor must be reported on pathology report of breast carcinoma b/c it affects what 3 things?
|
therapeutic options, prognosis and stage
|
|
|
What three findings are assessed by breast cancer histologic grade? Correlate grade to score.
|
tubule formation , nuclear atypia, number of mitoses (1-3 pts each); Grade I = 3-5, II = 6-7, III = 8-9
|
|
|
What tests can be done to assess for Her2-neu status in breast cancer? Which is better?
|
IHC for overexpression, FISH for gene amplification, has less false + and - results, should be done if IHC equivocal
|
|
|
What do the results of a Her2-neu test mean in breast cancer?
|
positive = more aggressive cancer, but also better response to Herceptin
|
|
|
What receptors can be tested for in breast cancer using immunohistochemistry? How are the results interpreted?
|
Presence of Estrogen and Progesterone receptors are a good prognostic indicator, better response to Tamoxifen
|
|
|
How is lymph node status determined in evaluating a breast cancer patient?
|
sentinel biopsy if no palpable node, axillary dissection if palpable or malignancy seen on sentinel biopsy
|
|
|
Why is the sentinal node the best place to biopsy if nodes are not palpable near a breast mass?
|
if negative, there is a very low likelihood of more distant node involvement
|
|
|
What treatment becomes an option for a breast mass if the sentinel lymph node biopsy is negative?
|
brachytherapy catheters for local radiation
|
|
|
What views are taken in a screening mammogram?
|
CC (cranial caudal) and MLO (medial lateral oblique)
|
|
|
What is a spot compression view of a breast mass and how is it interpreted?
|
Evaluates the margins (irregular is more likely malignant)
|
|
|
What can a magnification view tell you about a calcified lesion in a breast?
|
scattered or round, punctuate calcifications are usually benign; clusters, linear or pleomorphic suggest malignancy
|
|
|
What is the role of ultrasound in diagnosing breast cancer?
|
distinguish cysts (anechoic, smoothly marginated) from solid (hypopechoic, more likely malignant, esp if + irregular border)
|
|
|
What antiestrogenic therapies are available to a patient with ER+ cancer?
|
ovarian ablation, tamoxifen (partial agonist/antagonist), Fulvestrant (full antagonist) and Aromatase inhibitors (more effective and less toxic than Tamoxifen)
|
|
|
What changes occur in the cardiovascular system as a result of pregnancy?
|
increases in C.O., HR, and SV; Decreases in SVR b/c of progesterone; increased blood flow (more to renal, skin, breasts, uterus)
|
|
|
What cardiac changes occur during labor?
|
greater increase in CO due to uterine autotransfusion & and pain, valsalva leads to wide flux in BP and HR
|
|
|
What changes on cardiac assessment are normal for pregnancy?
|
HR < 100bpm, systolic efection murmur, S3, increased PVCs and PACs, prominent pulmonary vasculature
|
|
|
What type of valvular lesions are better tolerated in pregnancy?
|
regurgitant > stenosis
|
|
|
What complications occur in a pregnant patient who has an aortic stenosis?
|
b/c AS -> fixed Stroke volume, HR alone determines C.O.
|
|
|
What acid-base disturbances occur in pregnancy?
|
Progesterone increases sensitivity to CO2 -> hyperventilation. Respiratory alkalosis with metabolic compensation
|
|
|
What changes occur in the pulmonary system of a pregnant woman?
|
Increase in TV and RR (TV x RR = minute ventilation), at the expense of decrease in expiratory reserve
|
|
|
What is significant about the thoracic cage configuration in pregnancy?
|
enlarges overall, and this change happens earlier than can be accounted for by enlarging uterus
|
|
|
Why are there no changes in FEV1/FVC during pregnancy?
|
b/c there is no change in large airway function
|
|
|
What acounts for the dyspnea present in 60-70% of normal pregnancies?
|
reduced pCO2 and awareness of increased TV
|
|
|
What important points should you remember in treating a pregnant patient with an asthma attack?
|
"normal" pCO2 actually a sign of impending resp. failure (b/c in pregnancy pCO2 is 27-32), keep o2 sat above 95%, albuterol nebulizer can result in fetal tachycardia
|
|
|
What causes the relative hydronephrosis of pregnancy? On which side are these changes more prominent?
|
mechanical compression and smooth muscle relaxation (progesterone), RIGHT
|
|
|
Why is there a greater risk for pyelonephritis and complications during pregnancy? What are the complications?
|
urinary stasis, asymptomatic bacteriuria, comps: resp. compromise/ARDS, preterm labor
|
|
|
What changes occur in the renal physiology of a pregnant woman?
|
increased GFR, greater increase in RPF, therefore filtration fraction actually falls
|
|
|
What lab abnormalities are a result of the increased GFR in a pregnant patient?
|
lower serum concentrations of Cr and urea
|
|
|
Chronic renal insufficiency is a risk factor for what pregnancy complication?
|
preeclampsia
|
|
|
What hematologic changes result from the hemodilution of pregnancy?
|
anemia, thrombocytopenia
|
|
|
What changes occur in the immune system of a pregnant woman?
|
increased WBCs, alteration of T cells (more Th2, les Th1 and NK) toward Ab-mediated fxn
|
|
|
Because pregnancy causes a hypercoaguability state, the risk of what events increases? What prophylaxis is recommended?
|
DVT and PE, LMW heparin until 36 wks, then unfractionated
|
|
|
What changes occur in the GI tract as a result of pregnancy?
|
decreased motility, increased risk of GERD and gallstones, elevated ALP, decreased albumin and total protein
|
|
|
What accounts for the spider angioma and palmar erythema that are normal findings in pregnancy?
|
estrogen effects
|
|
|
What factors keep the uterus quiescent in pregnancy (until labor)?
|
progesterone activates PGDH (breaks down PGs locally) and NO -> cGMP prevents activation of clamodulin
|
|
|
What are the stages of labor?
|
0: uterine quiescence; 1: Uterine awakening, cervical dilatation; 2: active labor - from complete cervical dilatation to delivery of fetus; 3: from fetal delivery to last contraction
|
|
|
What happens during stage 1 of labor as a result of local PG increases?
|
PGs stimulate uterine muscle contractions (more in stage 2), increase gap jxns between uterine muscle cells, and help soften and thin the cervix
|
|
|
What results from the cortisol, estradiol and IL-1β increase during stage 1 of labor?
|
Ca++ channels, PGHS-2, connexin, PG and OXY receptors increase, K+ channels decrease
|
|
|
How does OXY result in uterine muscle contraction?
|
binds GPCR, activates PLC, increases in IP3 lead to Ca++ release, which activates calmodulin, stimulates myosin-actin binding
|
|
|
What physiologic changes in parturition occur as a result of increased cortisol? Where does the cortisol come from?
|
Fetal cortisol -> increased PG synthesis, lung maturation, pos feedback due to increased placental CRH
|
|
|
How are cytokines involved in the physiology of parturition? What are the normal and pathological ways this might occur?
|
SP-A induced macrophages release cytokines that act to upregulate PGHS-2, increased PGs (NL), infection induced macrophages could do the same pathologically
|
|
|
What is the limit of viability for a fetus and what accounts for this limit?
|
prior to 24 wks, there is no capacity for ventilation b/c lack air spaces and capillary network doesn't interact with rudimentary air spaces
|
|
|
What is the composition of the lung surfactant and what is its purpose?
|
90% phospholipid (decreases surface tension, keeps sacs partially inflated on exhalation increases compliance), protein 10%
|
|
|
What results from surfactant deficiency and who is at particular risk?
|
hyaline membrane disease, microatelectasis, poor compliance; preterm infants
|
|
|
What are the clinical signs of surfectant deficiency in an infant and what treatments are available?
|
Increased WOB: retrations, nasal flare, grunting, cyanosis on rm air, CXR: microatelectasis + air bronchograms; O2, CPAP, intubation + ventilation, replacement surfactant
|
|
|
What is transient tachypnea of the newborn?
|
failure of fluid resorption that normally occurs due to fetal adrenaline, maternal TRH, and glucocorticoids from both
|
|
|
What is the difference between primary and secondary apnea in a newborn?
|
Primary - stimulation initiates cry and breathing, secondary requires positive pressure. At birth, assume it's secondary and intervene
|
|
|
What things can cause failure to breathe in a newborn?
|
sedation (maternal), neuromuscular problems in the baby (hyptotonia, myopathies, spinal cord injury)
|
|
|
What things are assessed on Apgar score? In what order are the points lost?
|
color > response to suction > tone > respirations > heart rate (2 pts each)
|
|
|
What are Apgar scores good for? What should they not be used for?
|
Good, quick description of newborn; not used to determine need for resuscitation or to predict long-term outcome
|
|
|
Why is lung inflation key to cardiovasular transition in the newborn?
|
AO2 decreased pulm resistance, increased pO2 closes DA, pulm BF increases LA volume and closes FO
|
|
|
What is persistent pulmonary hypertension of the newborn and how does it develop?
|
Pulm vascular resistance remains high and leads to R to L shunt: pulm aplasia, antenatal closure of DA/nSAID use -> abnormal pulm musculature, constricted pulm vessels due to parenchymal lung dz
|
|
|
Why is it possible for R to L shunt to occur after the cardiovascular transition is complete at birth?
|
DA closure, FO flap closure are reversible and maintained by balanced systemic and pulm resistance
|
|
|
What are the normal transitional changes in vital signs for a newborn?
|
tachypnea and periodic breathing (w/o cyanosis), HR decreases from 150-180 at birth to 100-120 at 1 hr, murmurs are frequent (except in 1st minute after birth)
|
|
|
What RX to stop preterm labor would you pick on an upcoming test?
|
MgSO4
|
|
|
What population of newborns is at increased risk for neonatal hypoglcemia?
|
preterm or IUGR (no glycogen stores), IDM (insulin overproduction prolonged), polycythemia
|
|
|
What are the signs of neonatal hypoglycemia? How is the dx made?
|
JITTERY, irritable, lethargic, apnic or seizures; Blood glucoes < 45 w/sxs or < 35-40 w/o sxs
|
|
|
Why are neonates at high risk for hypothermia?
|
lg surface area to weight ration, wet, little fat (except brown fat, but premies don't have this)
|
|
|
If a full term baby can't maintain temp, what is the dDx?
|
infection and CNS dysfxn (premature and IUGR babies have trouble w/o these causes)
|
|
|
If a neonate develops jitteriness and seizures at 24-48 hours postnatally, and has a normal glucose level, what do you suspect?
|
hypocalcemia
|
|
|
After the initially feeding and long (6-12 hr) sleeping period, how often do most newborns want to eat?
|
every 2-3 hours
|
|
|
What should almost all healthy babies do by 24 hours?
|
pee and poop
|
|
|
What are the three types of disorders that are the main focus of prenatal diagnoses
|
chromosomal, single gene defects, anatomic abnormalities
|
|
|
How is a pregnancy dated?
|
from the first day of the last menstrual period, estimated date if parturition is 40 weeks after
|
|
|
When should the first prenatal visit ideally be? What assessments are done at this point?
|
6-8wks. Personal Hx, Fam Hx, racial background
|
|
|
What assessments are done during the first trimester for prenatal diagnosis?
|
CBC (MCV esp),
|
|
|
At what gestational age is a fetus tested for trisomy-21 and what tests are performed?
|
u/s, hCG, Inhibin A, UE3, Pregnancy Associated Placental Protein-A, MS-αFP; done at transition from 1st to 2nd trimester
|
|
|
What is αFP? How is it used to detect neural tube defects, gastroschisis, and down syndrome?
|
fetal albumin, made in liver - will leak out in neural tube defect and be high (2.5xnl) and low in Down Syndrome
|
|
|
What test is repeated late in the second trimester? Why?
|
αFP, neural tube defects
|
|
|
During the ultrasound done at 18-20 weeks, what aspects of the fetus are evaluated?
|
full anatomic evaluation - determines needs for cesarean delivery, pick up 2-3% who have major anomalies
|
|
|
At what stage is pregnancy termination no longer an option (in most states)?
|
24 wks, point of viability
|
|
|
How are the panel of Down's syndrome tests reported?
|
as a statistical chance of having a baby with Down Syndrome (1 in X)
|
|
|
What is so special about the age of 35 in pregnancy?
|
At this age the risk of amniocentesis resulting in miscarriage is equal to the likelihood of finding an abnormaility
|
|
|
What are the two ways to obtain access to fetal cells? How are they used to test for defects?
|
Amniocentesis and CVS; FISH detects aneuploidy and RFLP detects single gene defects
|
|
|
Why is advanced maternal age considered a risk factor for birth defects?
|
increased rate of non-dysjunction meiosis event, leading to aneuploidy
|
|
|
Targeted screening is offered to families with what known gene defects?
|
cystic fibrosis (hard b/c so many polymorphisms), Tay Sachs, hemoglobinopathies
|
|
|
Why is MCV important in diagnosing birth defects?
|
if less than 80%, most likely iron deficiency. If no iron deficiency present, could be thalassemia or sicke cell
|
|
|
Which fetal cell sampling method is more risky for complications?
|
CVS > amnio
|
|
|
What controls postpartum hemorrhage physiologically? What is an alternative Rx?
|
OXY, ergot derivatives if OXY doesn't work
|
|
|
What two drugs might be used conjuctively to induce labor?
|
Dinoprostone (PG gel) to dilate/efface the cervix, OXY to augment contractions
|
|
|
How does MgSO4 suppress uterine contractions?
|
Ca++ competition
|
|
|
What other drugs (besides MgSO4 that you're going to pick) inhibit labor? How?
|
terbutaline (block beta receptors on uterus), PG inhibitors would work but would close the ductus
|
|
|
What nSAID should be used in pregnancy?
|
acetaminophen
|
|
|
What drug should be re-dosed at pregnancy?
|
thyroid hormone
|
|
|
What drug can be used to induce fetal lung maturity? How is it administered?
|
betamethasone; transplacentally
|
|
|
What drugs approximate maternal levels in the breask milk?
|
ethanol, lithium, tertacycline (also weak compounds can get trapped because milk pH < blood)
|
|
|
What are the benefits of breastfeeding?
|
Immune protection to baby, nutritional composition tailored to baby's need, bonding/enhanced neuro., prevents post-partum hemorrhage, weight loss, economic, birth spacing
|
|
|
In what ways is breast milk nutritionally tailored to baby?
|
low solute content (immature kidneys) high poly-unsaturated fats, whey protein (rapid gastric emptying)
|
|
|
How many baby friendly hospitals are there in the world? In the U.S? In CO?
|
15000, 25, 1
|
|
|
What immune factors are present in the milk?
|
IgA, bioactive lipids (bind baby's GI tract), cellular (lymphocytic), lactoferrin
|
|
|
When does La leche league start titty-twisting a post-partum mom?
|
immediately after stage 3 of labor (they think of it as stage 4)
|
|
|
What might you look for if you see a large blood vessel supplying the edge of the placenta?
|
an accessory lobe left in the uterus
|
|
|
What is placenta previa and what are the clinical signs?
|
internal os of cervix partially or completely obstructed by placenta; painless bleeding and failure of fetal passage at labor
|
|
|
What is placenta accreta and what are the clinical signs? What predisposes to this condition?
|
trophoblast is more invasive into myometrium, results in maternal hemorrhage (usually 3rd trimester), scar or previa predisposes, percreta goes completely through the uterine wall
|
|
|
What happens as a result of retroplacental hemorrhage?
|
compresssion of fetus -> hypoxemia, abruption
|
|
|
What's the difference between chorioamnionitis and villitis?
|
Chorioamnionitis: ascending bacterial; Villitis: transplacental, CMV or Parvo
|
|
|
What are the signs of preeclampsia? What does it put you at risk for?
|
edema, proteinuria, hypertension (after 20wks); DIC, HELLP, abruption, seizures
|
|
|
What are the risk factors for preeclampsia?
|
African American ethnicity 1st pregnancy, twin, Fam Hx, obesity, Chronic TN, renal dz
|
|
|
What are the signs of fetal hydrops? What can cause this?
|
edema and effusion of body cavities; can be immune based, due to Parvo induced hemolysis, congenital heart problem
|
|
|
What is the most common tumor of the fetus and newborn? Is it usually malignant?
|
Sacrococcygeal teratoma, almost always benign
|

