![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
16 Cards in this Set
- Front
- Back
|
How to choose a solvent for recrystallization?
|
Compound not soluble at room temperature and is at higher temperatures.
For mixed pair, have one solvent that the compound is soluble in, and a miscible solvent that it isn't soluble. Dissolve compound, then add other solvent until cloudy and continue as regular |
|
|
How to recrystallize?
|
Dissolve compound in minimal hot solvent, hot filtration if necessary, cool to RT, cool on ice, vacuum filter, wash with cold solvent
|
|
|
Trimyristin extracted from nutmeg with what solvent? Recrystallized in what?
|
Diethyl ether
Recrystallized in hot acetone |
|
|
Cholesterol extracted from gallstones in what solvent? Recrystallized in?
|
Extracted in 2-propanol
Recrystallized in methanol and water solvent pair |
|
|
Experiment 3 - What conditions used to synthesize benzodiazepine?
|
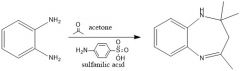
|
|
|
Clean NaCl IR plates with?
|
Chloroform, so the plates don't dissolve
|
|
|
Polarity of solvents are rated by what?
|
Dielectric constant, higher = more polar
|
|
|
What is retardation factor?
|
Rf = distance spot travelled/distance solvent travelled
|
|
|
What factors increase or decrease Rf?
|
The silica plate is polar. With a polar solvent, compounds will move up the plate faster. A non-polar compound should move faster. (higher Rf)
|
|
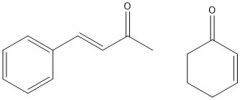
In experiment 4, two ketones were reduced with NaBH4. What were they reduced to?
|
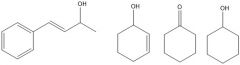
Because they are conjugated, there was a possibility that the C=C double bonds would get reduced too. The following products obtained:
|
|
|
In exp. 5, MS1 part A - p-aminophenol was acetylated. Draw scheme.
|
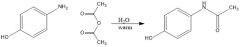
|
|
|
In exp. 6 MS1 part B - phenacetin was made from 4-hydroxyacetanilide. Scheme?
|
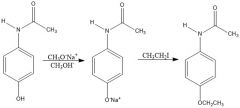
Using ethanol and sodium ethoxide minimizes hydrolysis of amide.
|
|
|
Mixed pair solvent and compound example?
|
Phenacetin in 95% methanol (minimal) and warm water (1.5x volume)
|
|
|
In ex 7 MS1 part C - phenacetin was brominated. Scheme?
|
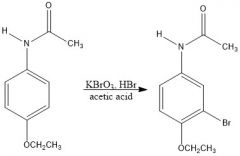
Generate Br2 in situ
|
|
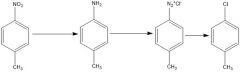
Fill in the conditions
|
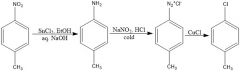
|
|
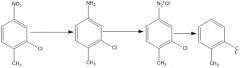
Fill in conditions
|
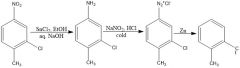
|

