![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
9 Cards in this Set
- Front
- Back
|
What are proteases / peptidases?
How are the two general types? |
A group of enzymes that catalyze the break down (hydolysis) of peptide bonds of proteins or peptides with the participation of a water molecule
Limited proteolysis - break specific peptide bonds depending on the aa sequence of a protein Unlimited proteolysis - break down a complete peptide to amino acids |
|
|
What are the two mechanism classifications of proteases?
|
Covalent catalysis:
a nucleophile in the protease leads to formation of an acyl-enzyme intermediate which hydrolyzes to free up the protease and the cleaved substrate (Ser, Thr, Cys proteases) General acid-base catalysis: an water molecule is activated to a nucleophile in a small cleft of the protease (Asp, Glt, metallo proteases) |
|
|
What are the substrate cleavage classifications of proteases?
|
1. Endopeptidase
- cleave the middle of the peptide 2. Exopeptidases - cleave the terminal amino acid of the substrate a. aminopeptidases: - have negatively charged side chains that bind to the positively charged amino terminus b. carboxypeptidases: - have positively charged side chains that bind to the negatively charged carboxyl terminus |
|
|
How does an endopeptidase locate the bind to be broken?
|
Subsites on the protease accomodate the side chain of each aa in the peptide chain, placing the peptide in the correct location to be cleaved by the enzyme
|
|
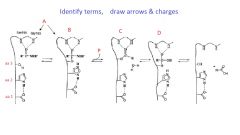
|
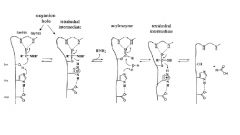
|
|
|
oxyanion hole
|
a pocket in the structure of an enzyme which stabilizes a deprotonated oxygen or alkoxide, often by placing it close to positively charged residues
|
|
|
What is the role of Asp in the serine protease mechanism?
His? Ser? |
Asp: the carboxylate in Asp increases the basicity of the imidazole moiety in His
His: the imidazole in His increases the nucleophilicity of the Ser OH Ser: the OH in Ser serves as nucleophile to react with the amide carbonyl group |
|
|
Define: serpins
|
an acronymic name given to a family serine protease inhibitors that share a complex tertiary structure
|
|
|
What is PMSF?
Draw structure and mechanism |
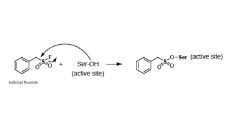
(phenylmethyl)sulfonyl fluoride
- an irreversible serine protease inhibitor Can inhibit many serine proteases as well as cysteine proteases |

