![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
43 Cards in this Set
- Front
- Back
|
A solution that contains equal numbers of hydrogen ion and hydroxide ions is?
|
Neutral
|
|
|
A peptide bond is formed
|
When two amino acids are bonded together
|
|
|
The phosphate portion of a phospholipid is
|
Polar and can form hydrogen bonds with water molecules
|
|
|
Approximately 96% of the body's mass is composed of the elements
|
C, H, O, N
|
|
|
One of the most important and most abundant inorganic compounds in the body is
|
Water
|
|
|
A substance with a pH of 5
|
Has fewer H+ than a substance with a pH of 3
|
|
|
Cellulose is a
|
Polysaccharide
|
|
|
If the outer electron shell is complete, the atom is
|
Chemically Stable
|
|
|
A covalent bond is formed
|
When atoms share one or more electrons
|
|
|
Which of the following are disaccharides?
A. glycogen, starch B. glucose, fructose C. cholesterol, triglyceride D. sucrose, lactose |
D. Sucrose, Lactose
|
|
|
Substances that ionize in water are called
|
Electrolytes
|
|
|
Which of the following represents a decomposition reaction?
A. Na+ + Cl- ----->NaCl B. NaOH + HCl -----> HOH + NaCl C. sucrose -----> glucose + fructose D. C + D -----> AB |
C. sucrose -----> glucose + fructose
|
|
|
The symbol Na+ represents a sodium atom that has lost
|
An Electron
|
|
|
An ionic bond is formed
|
When one atom gains an electron and one atom loses an electron in the formation of a bond
|
|
|
The first electron shell (nearest the nucleus of an atom) holds a maximum of
|
2 Electrons
|
|
|
The smallest unit of matter that retains the properties and characteristics of the element it makes up is called
|
An Atom
|
|
|
Which element is a component of all protein molecules, DNA and RNA?
A. potassium B. calcium C. sodium D. nitrogen |
D. Nitrogen
|
|
|
If sodium loses an electron, it becomes a(n)
|
Cation
|
|
|
The likelihood that an atom will form a chemical bond with another atom depends on
|
The number and arrangement of electrons in the outermost shell
|
|
|
Helium is a chemically stable gas because
|
Its outermost shell is completely filled with electrons
|
|
|
The most common molecule in the body that has polar covalent bonds is
|
Water
|
|
|
The collision energy required to break the bonds of the reactants is called its
|
Activation Energy
|
|
|
An increase in temperature will increase the rate of a chemical reaction because it increases the _________ of the substances involved
|
Speed
|
|
|
In a solution, a substance called the __________ dissolves another substance called the __________ .
|
Solvent; Solute
|
|
|
Water is an excellent solvent because
|
It has polar covalent bonds
|
|
|
Because water requires a large amount of heat to change from a liquid to a gas, it
|
Provides an excellent cooling mechanism
|
|
|
A polysaccharide that is found in muscle and liver cells is
|
Glycogen
|
|
|
Which of these substances would form an acid when dissolved in water?
A. NaCl B. NaOH C. KCl D. HCl |
C. KCl
|
|
|
Some molecules are said to be amphiphatic because they
|
Have both polar and nonpolar regions
|
|
|
One end of a phospholipid is hydrophilic because
|
The head is a polar phosphate group
|
|
|
The building blocks of proteins are
|
Amino Acids
|
|
|
All steroids contain
|
Four rings of carbon atoms
|
|
|
If a protein encounters a change in temperature, pH, or electrolyte concentration it will
|
Unravel and lose its characteristic shape
|
|
|
The portion of an enzyme where the substrates attach is called the
|
Active Site
|
|
|
The names of enzymes usually end with the suffix
|
-ase
|
|
|
The chemical called the energy currency of living systems is
|
ATP
|
|
|
Which of the following is the smallest in size?
A. an ion B. an electron C. a neutron D. an atom |
B. an electron
|
|
|
Atoms of different elements are distinguished from each other by their:
|
Atomic Number
|
|
|
When a peptide bond is formed, a molecule of water:
|
Is Removed
|
|
|
Enzymes bind only to reactant molecules called:
|
Substrates
|
|
|
A major function for carbohydrates is:
|
As a readily available source of energy
|
|
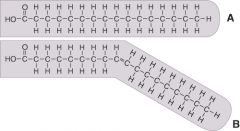
Which of the fatty acids is unsaturated?
|
B
|
|

Which of the beakers contain a basic solution?
|
B
|

