![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
116 Cards in this Set
- Front
- Back
|
What are the two immune system defense mechanisms?
|
Innate (aka natural or native immunity)
Adaptive (aka specific immunity) |
|
|
Does the innate immune system have memory?
|
No
|
|
|
Innate responses are mediated through which receptors?
|
Toll-like receptors (TLR) - coordinate various cytokine-generated, complement-mediated, and phagocytic responses
|
|
|
Portions of molecules that are recognized by lymphocytes are known as ___________ or __________
|
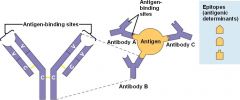
determinants or epitopes
|
|
|
Adaptive responses are mediated through which receptors?
|
Antigen-specific receptors on the surfaces of T and B lymphocytes & antibodies
|
|
|
The total number of antigenic specificities recognized by lymphocytes is known as the __________________.
|
lymphocyte reservoir
|
|
|
Does the adaptive immune system have memory?
|
Yes, responses to subsequent exposures are more rapid and pronounced than initial responses
|
|
|
The state where there is immunologic unresponsiveness to an antigen is known as what?
|
Tolerance - abnormalities in the induction or maintenance of tolerance to self lead to autoimmunity and the induction of a range of dz's
|
|
|
What are the principle cells that are part of the adaptive immune system?
|
Lymphocytes - T & B cells
Accessory cells - macrophages, dendritic cells (APC) Effector cells - activated T lymphocytes, macrophages |
|
|
What are the two types of adaptive immunities?
|
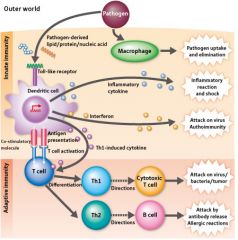
Adaptive Immunity:
1) Humoral Immunity - mediated by B lymphocytes and their secreted antibodies 2) Cellular (cell-mediated) immunity - mediated by T lymphs |
|
|
List the organs make up the primary lymphoid system where adaptive immune responses take place?
|
Thymus
Bone Marrow Lymph Nodes Spleen Cutaneous immune system Mucosal immune system (Respiratory & GI) |
|
|
Where is the site of T-cell maturation?
|
Thymus
|
|
|
What are thymocytes?
|
T-cells at various stages of maturation in the thymus
|
|
|
Where is the site of B-cell maturation?
|
Bone marrow
|
|
|
Where is the location of B, T and plasma cells within the following lymph node areas?
Cortex Paracortex Medulla |
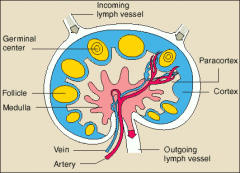
Cortex - B cells
Paracortex - T cells & APC Medulla - B, T and plasma cells |
|
|
Which organ is the site of immune responses to blood-borne antigens?
|
Spleen
|
|
|
In the spleen, lymphoid follicles surround small arterioles in the _______________________________.
|
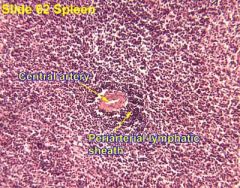
Periarteriolar lymphoid sheaths (PALS)
|
|
|
What are Langerhans cells in the skin?
|
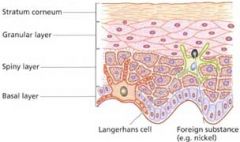
Are immature dendritic cells found in the skin and are active in the processing of Ag detected by the cutaneous immune system.
As Ag's are captured by Langerhans cells, under the influence of proinflammatory cytokines, these cells migrate into the dermis and then to the regional lymph nodes for stimulation of a specific immune response. |
|
|
Mucosa of GI tract demonstrates scattered collections of lymphocytes in clusters known as __________________.
Which type of cells are typically found there? |
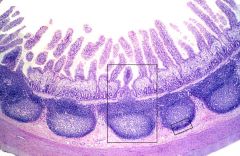
Peyer's patches
T-cells |
|
|
What type of epithelial cells are found in tonsils and adenoids?
|
M (membranous/microfold) cells
|
|
|
What is the major immune function of tonsils and adenoids?
|
1) Generation of antigen-specific B cells in the tonsillar follicles
2) Production of secretory IgA |
|
|
Lymphocytes are responsible for two primary functions of the adaptive immune system, ______________ and __________________
|
specificity and memory
|
|
|
What are naive lymphocytes?
|
lymphocytes that have not been previously stimulated by Ag
|
|
|
Which lymphocyte is involved in humoral immunity and which is involved in the cell-mediated immunity?
|
B-cells = humoral immunity (antibody production)
T-cells = cellular immunity (cell-mediated) (note - natural killer cells are mainly involved in the innate response) |
|
|
T helper cells have which CD count?
|
CD4
Induce cytotoxic or suppressor T cells (CD8 cells) Mature from naive CD4 cells (Th0) into either Th1 or Th2 |
|
|
Th1 cells participate in which immunity?
Which factors are produced by these cells? Do they stimulate or inhibit B cells? |
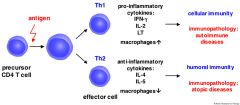
Th1 cells participate in microbial/cell-mediated immunity
Produce IL-2 and interferon gamma when activated Inhibit B cells |
|
|
Th cells participate in which immunity?
Which factors are produced by these cells? Do they stimulate or inhibit B cells? |
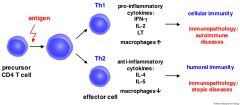
Participate in the allergic & humoral responses
Produce IL4, 5, 6 and 10 Stimulate B cells & also recruitment and activation of eosinophils |
|
|
What are Th17 cells?
|
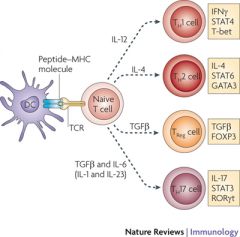
Recently characterized third subset of T helper cells
- Respond to host infections with specific bacterial and fungal species - Involved in the recruitment and activation of neutrophils - Create profound local inflammatory changes - Appear to be involved in autoimmune dz's such as MS |
|
|
What are Treg cells?
|
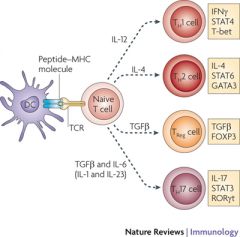
Another T-cell subset known as T regulatory (Treg) cells
- Regulate immune responses in vivo - Exert suppressor effects on effector T cells and APC's - Involved in prevention of autoimmunity - Involved in induction if immune tolerance - Play a central role in therapeutic effects noted with allergy immunotherapy |
|
|
Natural Killer cells are positive for CD__
|
CD16
|
|
|
What are major histocompatibility complex proteins?
|
Specialized proteins responsible for displaying antigens for recognition of antigens by and presentation of peptides to T cells.
Principle function is to bind fragments of foreign proteins, thereby forming complexes that are recognized by T cells. |
|
|
What are the two main types of MHC and where are they found?
|
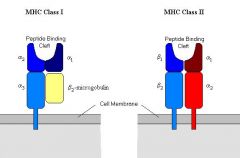
MHC Class I - membrane-associated glycoproteins present on nearly all nucleated cells
MHC Class II - normally expressed on on B-cells, macrophages or dendritic cells, endothelial cells and a few other cells lines. |
|
|
What is the role of interleukins?
|
Cytokines produced by leukocytes that act on other leukocytes
|
|
|
Principle Cytokines Involved in the Allergic Responses
IL2 What's the function? Derived from what cells? |
IL2 is a growth factor for antigen-stimulated T cells and responsible for T-cell clonal expansion.
It promotes the proliferation and differentiation of other immune cells. Derived from T-cells. |
|
|
Principle Cytokines Involved in the Allergic Responses
IL3 What's the function? Derived from what cells? |
IL3 promotes development and expansion of immature bone marrow elements.
Promotes mast cell proliferation Promotes eosinophil activation Derived from CD4+ T-cells |
|
|
Principle Cytokines Involved in the Allergic Responses
IL4 What's the function? Derived from what cells? |
Principle cytokines that stimulates B-cells isotypes switching to the IgE isotype (for allergic rxns)
Stimulates the development of Th2 cells from naive CD4 cells Derived from CD4+ T-cells and mast cells |
|
|
Principle Cytokines Involved in the Allergic Responses
IL5 What's the function? Derived from what cells? |
Activate eosinophils and links T-cell activation and eosinophilic inflammation
Stimualtes the growth of eosinophils and activation of mature eosinophils Involved in stimulating eosinophil chemotaxis to areas of inflammation Derived from CD4+ T-cells |
|
|
Principle Cytokines Involved in the Allergic Responses
IL10 What's the function? Derived from what cells? |
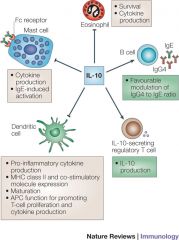
Inhibits activated macrophages
Inhibits cytokine synthesis in various cells Maintains homeostatic control of immune rxns Suppresses APC's Acts as an important regulator of the immune system Derived from CD4+ T-cells and activated macrophages |
|
|
Principle Cytokines Involved in the Allergic Responses
IL13 What's the function? |
Functions in a similar manner as IL4 (helps with IgE)
Induces adhesion molecules at sites of allergic inflammation contributing to increased populations of eosinophils and lymphocytes. |
|
|
Give a quick overview of cell-mediated immunity, starting with the antigen
|
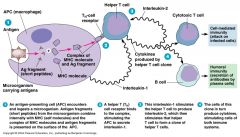
- APC's (ie. dendritic cells) recognize the Ag & present it to T-cells in association with Class II MHC molecules.
- T-cell activation occurs, which then causes T-cell proliferation, differentiation and production of cytokines and various effector functions. - Migration of activated T-cells and other leukocytes to sites of inflammation |
|
|
Which chain on the Ab, light or heavy, binds to host tissues and complement and determines Ig class?
|
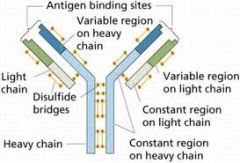
Heavy chain
|
|
|
Which region of the Ab is constant, and which is variable?
|
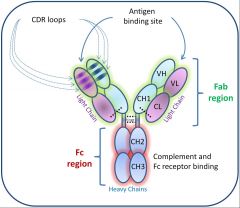
Constant region (C-terminal end of Ig molecule) or Fc fragment
Variable region (N-terminal end) of Fab fragment |
|
|
Which is the only Ig molecule that can cross the placenta?
|
IgG
|
|
|
Which Ig has a pentamer shape?
|
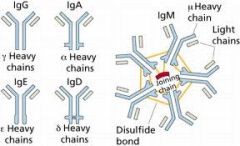
IgM
|
|
|
List the four types of hypersensitivity reactions
|
Type I - anaphylactic
Type II - cytotoxic Type III - immune complex mediated Type IV - delayed (cell-mediated) |
|
|
What is the mechanism of Type I hypersensitivity rxn?
|
Type I hypersensitivity rxn = anaphylactic
1) Cross-linking of IgE molecules on mast cells 2) Degranulation of mast cells 3) Release of histamine |
|
|
Which type of hypersensitivity do the following portray?
Hemolytic anemia Transfusion rxn Hyperacute graft rejection Goodpasture's syndrome Myasthenia gravis |
Type II - Cytotoxic
|
|
|
What is the mechanism of Type II hypersensitivity rxn?
|
Type II hypersensitivity rxn - cytotoxic
IgG or IgM mediated Ab react with antigens on cell membranes Activation of compliment occurs |
|
|
Which type of hypersensitivity do the following portray?
Serum sickness Post-streptococcal glomerulonephritis Arthus rxn Angioedema GI intolerance |
Type III - immune complex mediated
|
|
|
What is the mechanism of Type II hypersensitivity rxn?
|
Immune complexes are formed, usually with IgG Ab's
Complexes deposited in tissues & activated Initiation of acute inflammatory response |
|
|
Which type of hypersensitivity do the following portray?
Acute/chronic dermatitis Granulomatous dz's like TB and sarcoidosis Fungal dz's |
Type IV - Delayed (cell-mediated) hypersensitivity
Mediated by direct T-cell activation Cell-mediated inflammation |
|
|
The susceptibility to develop an allergic response is know as _______
|
atopy - implies a genetic predisposition toward the expression of allergic dz
|
|
|
Atopic individuals demonstrate a skewing of T helper responses to the ____ pattern.
|
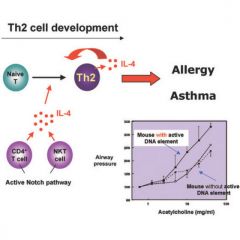
Th2 - remember that Th2 produce IL4, which is the main interleukin stimulating eosinophil production
|
|
|
What are the tissue effects of histamine?
|
Histamine is the main mediator of the allergic rxn
1) Vasodilation 2) Increased capillary permeability 3) Bronchoconstriction 4) Tissue edema |
|
|
What is the pathophys of Allergic shiners & Dennie's lines?
|
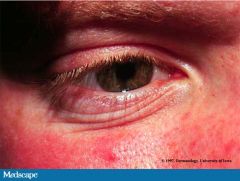
Allergic Shiners - darkening under the eyes from chronic deposition of hemosiderin in tissues
Dennie Morgan lines - fine horizontal lines in the lower lids; occur through spasms of Mueller's muscles in the lids |
|
|
What is the cause of "cobblestoning" of the posterior pharyngeal wall?
|
Presence of lymphoid aggregates or patches on the posterior pharyngeal wall
|
|
|
What is the time frame of early and late phase allergic responses?
|
Early phase - within 5-10 minutes
Late phase - begins 4-8 hours after exposure; can last for 24hrs or more. |
|
|
What are the primary mediators of early and late phase allergic responses?
|
Early - primarily histamine mediated
Late - primarily mediated by newly generated mediators of inflammation and cellular infiltration (leukotrienes & eosinophils) |
|
|
What are the four broad categories of allergic triggers?
|
Inhalants (pollen, danders, molds)
Ingestants (foods, meds) Injectables (meds, insect venom) Contactants (nickel, poison ivy, meds) |
|
|
Which seasons are the following pollens typically present in?
Tree pollen Grass pollen Weed pollen |
Tree pollen - winter & spring (Feb-May)
Grass pollen - later spring and summer (Apr-Aug) Weed pollen - later summer & fall (Jul-1st frost) |
|
|
What is ARIA and what are it's categories based on?
|
ARIA = Allergic Rhinitis and its Impact on Asthma; guidelines set forth by WHO
Categories based on chronicity and severity of symptoms |
|
|
What are the categories of ARIA, and what are the guidelines for those categories?
|
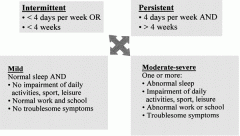
Chronicity categories:
1) Intermittent - <4 days per week OR <4 wks per yer 2) Persistent - 4 or more days per week AND > 4wks per yer Severity categories: 1) Mild - no impact on quality of life or fnct 2) Moderate or severe - sx impact quality of life (ie. work, sleep, and school) |
|
|
What are Thommen's postulates?
|
Antigenicity of a pollen is determined by Thommen's postulates.
Proposed in 1931 by A.A. Thommen, it is five characteristics a pollen must have to be considered allergenic: 1) Windborne 2) Light enough to be carried large distances in the air 3) Produced in large quantities 4) Abundantly distributed in the environment 5) Able to be allergic to sensitive individuals |
|
|
What are the two primary dust mite species responsible to US allergic dz?
|

Dermatophagoides pteronyssinus
Dermatophagoides farinae |
|
|
What are the primary antigenic proteins found in dust mites?
|
DerP1 (for D. pteronyssinus)
DerF1 (for D. farinae) |
|
|
What is the primary antigen found in cat's & dogs?
|
Cats = FelD1
Dogs = CanD1 |
|
|
Molds may promote which type of hypersensitivity reaction(s)?
|
Type I & IV
|
|
|
What are the leading mold offenders causing allergy?
|
Aspergillus
Alternaria Curvularia Hermodendrum Cladosporium Pullaria Penicillium |
|
|
Diagnosis of allergy involves which three elements??
|
1) History
2) Physical examination 3) Diagnostic testing |
|
|
Diagnostic Testing of the Allergic Patient
What is the nasal cytology designed to evaluate? |
For the presence of eosinophils vs neutrophils in the nasal mucus.
Poorly sensitive & specific |
|
|
What are the two classifications of in vivo allergy tests?
|
Epicutaneous & percutaneous
|
|
|
What are the various epicutaneous techniques?
|
Scratch, prick and puncture tests
Introduce the antigen into the epidermis only |
|
|
How do perutaneous techniques work?
|
Introduce the antigen into the superficial dermis
|
|
|
Describe the scratch test, including advantages & disadvantages.
Is it recommended for office use? |
Epicutaneous method
Two millimeter superficial lacerations are made in the skin & a drop of concentrated Ag is applied to the scratch. Usually conducted on the pt's back Advantages 1) Generally safe 2) Systemic rxns are uncommon 3) Rare delayed skin rxns 4) Large surface area for testing Disadvantages 1) False-positive rxns common 2) Related to traumatic rxns of the skin rather than immune-mediated allergic rxns 3) More painful than other types of testing 4) Tests are poorly reproducible Because of variable sensitivity, poor specificity and lack of reproducibility, scratch testing is NOT currently recommended as a dx procedure for assessment of inhalant allergy. |
|
|
Describe the prick or puncture tests, including advantages & disadvantages.
Is it recommended for office use? |
Epicutaneous technique (epidermis only)
Most widely used allergy testing method worldwide. Antigen is placed at the site of skin prick , and 10-20 min are allotted for the skin to react. Measurement of the reaction can be done through a subjective system (0-4+) or through direct measurement of wheal diameter. Advantages 1) Rapid & Easy 2) Interrater consistency is good 3) High degree of safety (rare systemic rxns) 4) Excellent screening test for the presence or absence of allergy. Disadvantage 1) Allows only a qualitative assessment of allergen sensitization. 2) May be less sensitive to low degrees of allergy sensitization (can result in false-negatives) 3) Grading of skin response is less objective than with intradermal techniques. Excellent screening method for the presence of absence of allergy to individual antigens. |
|
|
What are the two types of intradermal allergy testing?
|
single and multiple-dilutional techniques
|
|
|
In a multiple-dilutional intradermal allergy testing, a wheal size of ___ mm is considered a positive whealing response.
|
7mm
|
|
|
Intradermal dilutional testing (IDT) was formerly referred to as what?
|
skin endpoint titration (SET)
|
|
|
Which allergy testing method uses blended epicutaneous or percutaneous technique?
|
Modified quantitative testing (MQT)
|
|
|
Which skin allergy test produces quantitative information regarding degree of allergic sensitization without the need to complete a full IDT battery?
|
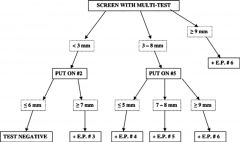
Modified quantitative testing (MQT)
|
|
|
First generation antihistamines can suppress the wheal response for how many days if taken prior to testing?
How about second generation antihistamines? |
2-3 days
7 days (some up to 10 days, like desloratadine) |
|
|
How do tricyclic antidepressants affect the wheel & flare response?
|
Have antihistaminic properties
May suppress the wheal and flare response for up to 7 days |
|
|
How do leukotriene receptor antagonists (ie. montelukast) affect the wheel & flare response?
|
Have NOT been shown to suppress wheal & flare in several trials.
May hold for 7 days if suspected to have suppressed wheal and flare in a specific patient. |
|
|
How do topical and oral steroids affect the wheel & flare response?
|
No affect on wheal & flare
|
|
|
What's the problem with using topical or oral beta-blockers during allergy testing?
|
Beta-blockers increased resistance to epinephrine (used as a rescue medication in case of anaphylaxis).
Also, if you do have to use epi in a patient who is on beta-blockers, the unopposed alpha stimulation can lead to critical HTN and arrhythmia. |
|
|
When was the original in vitro assay developed?
|
In 1970; RAST = radioallergosorbent test
|
|
|
Summarize the classic RAST technique
|
RAST measures the serum level of allergen-specific IgE
Technique 1) Ag-bound paper disk is used for assessment 2) Disk is placed in a test tube, to which pt's serum is added 3) Allergen-specific IgE present in serum binds to Ag on the disk. 4) Radiolabeled anti-IgE Ab is added & binds to the pt's IgE bound to the Ag on the disk 5) The amount of bound radiolabel is then measured with a gamma counter |
|
|
Summarize the changes in a modified RAST technique
|
Called mRAST
1) Specific IgE Ag's can be presented in other formats (ie. solid phase, three-dimensional) 2) lLbels other than radioactive labels can be used (fluorescent labels, enzyme-lined assays) 3) Length of time for Ag to interact with serum can be varied |
|
|
Can total IgE measurements be used to diagnose allergy?
|
Total IgE can be useful as a screen (ie..>2000 IU suggests allergy), but low levels do not necessarily eliminate the presence of a clinically significant allergy
|
|
|
What kind of allergy testing can you do with patient who is on beta-blockers?
|
In vitro testing (ie. RAST)
|
|
|
The treatment of allergy generally relies upon which three-pronged theory?
|
1) Environmental control
2) Pharmocotherapy 3) Immunotherapy |
|
|
Antihistamines used for allergies affect which receptors?
|
H1-receptor antagonists that act mainly by dose-dependent competitive binding of H1 receptors on target cells in the eyes, nose, skin and lungs
|
|
|
Describe some characteristics of first generation oral antihistamines
|
Developed in 1940
-Highly lipohilic (crosses the BBB & causes sedation & CNS suppressive effects) -Highly anticholinergic (drying of mucus mebranes, increase in mucus tenacity, blurring of vision, constipation, urinary retention) -May be accompanied with tachyphylaxis |
|
|
Describe some characteristics of second generation oral antihistamines
|
Developed in 1980
- Lipophobic (does NOT cross the BBB; less likely to cause sedation & CNS effects, but dose related) - Little or no anticholinergic activity (safe to use in pts with asthma, and little if any tachyphylaxis) |
|
|
Decongestants work on which receptor (be specific)?
|
α2 agonists - cause vasoconstriction
|
|
|
What are some side effects of using systemic decongestants (pseudoephedrine and phenylephrine)?
|
Can be accompanied with significant alpha-adrenergic SE:
1) Increased BP in HTN pt's 2) Increased appetite 3) Increased cardiac symptoms (tachy & arrhythmias) Can also infrequently be accompanied with significant tachyphlaxis (rebound htn) |
|
|
Explained the pathophys of rhinitis medicamentosa
|
Rapid rebound congestion developing with use of topical decongestant use for as few as 3 days. With continued use, the tissue becomes less responsive to the medication, requiring more frequent usage of the sprays with a diminished effect. If this vicious cycle is continued, permanent damage to the nasal lining may occur.
|
|
|
What is the only topical nasal corticosteroids with a Pregnancy Class B?
|
Budesonide (Rhinocort Aqua)
|
|
|
What is the MOA of cromolyn sodium?
|
Stabilizes mast cell membranes and inhibits degranulation and release of histamine
|
|
|
What are some disadvantages of cromolyn use for allergic rhinitis?
|
Must be administered 3-4x daily due to very short half life
Will not tx sx once exposure has occurred and mast cells have degranulated = needs to be used prophylactically Efficacy is mild to moderate at best |
|
|
Which leukotriene receptor blockers is the only agent approved in the tx of allergic rhinitis?
It is indicated for use down to what age? |
Montelukast
Indicated for use down to 6 months of age |
|
|
Immunotherapy generally is felt to require at least how many yrs?
|
3-5 yrs of regular administration to permit persistence of benefits after discontinuation
|
|
|
Immunotherapy causes an increase in specific IgG4 levels of treated allergens. What is IgG4?
|
Formerly thought to induce immune tolerance as blocking antibodies
Probably more a marker of T helper regulation than a true mediator of effect |
|
|
Explain how subcutaneous immunotherapy (SCIT) works
|
Injections of sequentially greater concentrations of allergens over time to induce hyposensitization and immune tolerance
Begins with very dilute concentrations of allergenic sera, injected once or twice weekly, and advanced until maximal tolerability and delivery of adequate dosage of antigen is achieved. Starting doses should be high enough to rapidly induce an immune response, but low enough to avoid any significant local or systemic adverse effects. |
|
|
What is the purpose of dose escalation in subcutaneous immunotherapy?
|
To achieve the maintenance dose, which is the target dose where maximal antigen is delivered with each dose, yet w/o systemic or large local reactions.
Maintenance dose should deliver a targeted cumulative level of antigen when given over a period of 3-5 yrs. |
|
|
In subcutaneous immunotherapy, what is the safe dose increase?
|
Generally, increasing 0.05 - 0.10 of antigen WEEKLY is safe and tolerated well.
|
|
|
How do you know when maintenance dose of subQ immunotherapy has been achieved?
|
There is no specific level that is appropriate for all pt's
Goal is to administer the highest tolerated concentration of antigen per injection A maintenance injection should control sx for about 1 weeks Local reaction of 3-4cm would suggest that maintenance dose is achieved |
|
|
Once maintenance dose is achieved with subQ immunotherapy, can you space out the dosing intervals?
|
Dosing intervals can be increased from weekly to every 2-3 weeks after 6-12 months of injections and if sx are adequately controlled between injections
|
|
|
When do you stop immunotherapy?
|
Most pt's are able to stop SCIT in 3-5yrs AFTER achieving maintenance dose
|
|
|
In anaphylaxis, what are the adult and pediatric doses of epi?
|
Adult - 0.3cc SubQ
Children - 0.1 to 0.2cc SubQ |
|
|
Which immunotherapy is an alternative to subcutaneous?
|
Sublingual immunotherapy (SLIT)
|
|
|
Why is sublingual immunotherapy not used as much in US?
|
Mostly due to lack of FDA approval for antigen use sublingually, and lack of US studies demonstrating efficacy.
SLIT has been used in Europe over the past 50 years with excellent results. |
|
|
What is a food allergy?
|
IgE mediated reaction to known allergenic foods
There are non-IgE-mediated food reactions, but these are poorly understood and characterized. |
|
|
There appears to be a genetic predisposition to the development of food allergy...what are they?
|
Predominance of Th2 lymphocytes
Increased production of IL4 and IL5 Enhanced mast cell releasability Lack of immune-protective factors Increased Ag resorption 2*/to loss of mucosal integrity |
|
|
Do elevated specific IgG levels imply allergy?
|
No, only exposure to those foods in the diet. Not suggestive or diagnostic of food allergy.
|
|
|
What is the gold standard for diagnosing food allergy?
|
Double-blind placebo-controlled food challenge (DBPCFC)
|

