![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
53 Cards in this Set
- Front
- Back
|
Mixed Non Competitive |
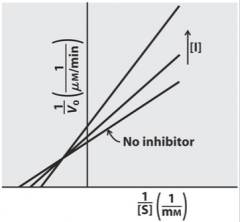
|
|
|
Uncompetitive Competition
|
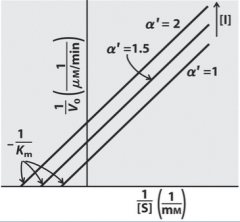
|
|
|
Non Competitive |
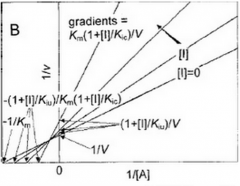
|
|
|
Competitive |
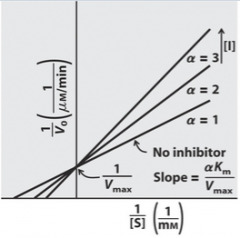
|
|
|
y-axis |
1/Vo |
|
|
x-axis |
1/[S] |
|
|
KM |
1/2 Vmax, x-value |
|
|
pseudo first order kinetics |
within the box of KM |
|
|
Vmax |
max velocity, max amount of speed the enzyme can work out. |
|
|
Zero order kinetics |
Vmax, rate is independent of concentration of the reactant (substrate) |
|
|
When does M-M apply? |
-unimolecular reaction or bimolecular when the [one] does not change - irreversible rxn (rare) or [P] = 0 initial cond. -concentration of substrate is vastly different than E total also [E]T |
|
|
First Order Kinetics Equation |
Vo= k[s]1 , Vo proportional to [S]o |
|
|
Zero Order Kinetics Equation |
V= k [s]0 |
|
|
Intracellular Conditions |
Constant: Temp, PH, Concentration, Pressure |
|
|
Metabolic Pathways |
The feedback inhibitor (the end enzyme) inhibits the first possible enzyme in the pathway. |
|
|
allosteric site |
an inhibitor attaches to the allosteric site and thus closes the active site of the protein |
|
|
Catalytic Power |
rate w/enz / rate w/o enz |
|
|
Regulation |
ability to be turned on and off |
|
|
specificity |
for both rxn and reactant/ prods |
|
|
Branched Pathways |
two different pathways |
|
|
anabolic |
use small metabolites and enzymes source build macromolecules |
|
|
catabolic |
going to breaking down macromolecules |
|
|
specificity |
only fit into specific substrate of a specific protein also only specific for a specific reaction (catalytic side chains on enz arranged in space) |
|
|
ligases
|
reactions in which new bonds are formed between carbon and another atom; energy is required |
|
|
isomerases |
reactions in which a compound is converted to its isomer |
|
|
lyases |
reactions in which groups are removed without hydrolysis or addition of groups to a double bond |
|
|
hydrolases |
hydrolysis reactions |
|
|
transferases |
transfer reactions of groups, such as methyl, amino, and acetyl |
|
|
oxidoreductases |
oxidation-reduction reactions |
|
|
Random Single Displacement |
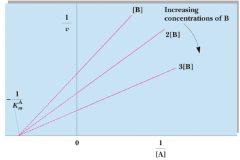
|
|
|
Ordered Single Displacement |
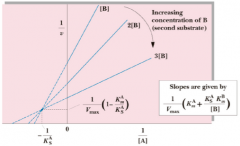
|
|
|
Double Displacement or Ping Pong |
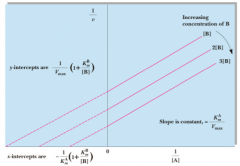
|
|
|
x-intercept Displacement |
-1/Ks |
|
|
x-intercept of Ping-Pong |
-1/Kam (1+ Kbm/[B] |
|
|
holoenzyme
|
catalytically active complex of protein and prosthetic group
|
|
|
apoenzyme
|
protein without the prostheticgroup; it is catalytically inactive.
|
|
|
prosthetic groups
|
a coenzyme is firmly associated with its enzyme, perhaps evenby covalent bonds, and it is difficult to separate the two
|
|
|
A ----> I ----> J ----> P Which enzyme will be the inhibitor and which enzyme will be inhibited?
|
P will inhibit A |
|
|
unimolecular reactions
|
the molecularity equals 1, first order reactions
|
|
|
bimolecularreactions
|
its molecularity is 2, where two molecules must react to yield products, 2nd order reactions, rarely found
|
|
|
second-order
|
first-order with respectto A and first-order with respect to B
|
|
|
second-order rate law |
v = k[A][B] or v = [A]^2 |
|
|
first-order rate law |
v = k[A] |
|
|
transition state
|
the probability is very high that theparticular rearrangement accompanying the A---->P transition will occur
|
|
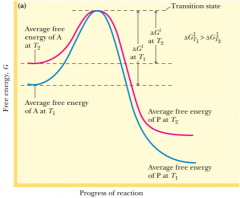
|
raising the temperature brings the activation energy up to the needed delta G double dagger so the reaction can be performed |
|
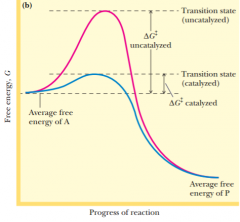
|
Catalystswork by lowering the energy of activation rather than by raising the average energyof the reactants. Catalysts accomplish this remarkable feat by combiningtransiently with the reactants in a way that promotes their entry into the reactive,transition-state condition. |
|
|
Rates are doubled when temperature is increased at increments of ______ degrees C. |
10 |
|
|
Vmax |
At high [S], v becomesvirtually independent of [S] and approaches a maximal limit.
|
|
|
saturation effect
|
When v shows no increase even though [S] is increased, the system is saturated withsubstrate.
|
|
|
active site
|
that place on the enzyme where S binds
|
|
|
steady-state assumption |
That is, ES is formed asrapidly from E + S as it disappears by its two possible fates: dissociation to regenerate E + S and reaction to form E + P. That is, the change in concentration of ES with time, t, is 0.
|
|
|
equation for back reaction |
v = k-2[E][P]
|
|
|
initial velocity
|
is only observed in the reactionimmediately after E and S are mixed in the absence of P (back reaction) |

