![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
58 Cards in this Set
- Front
- Back
|
bondings groups: 2 lone pairs: 0 |
Electron geometry: Linear Molecular geometry: Linear Bond Angles:180 |
|
|
bondings groups: 3 lone pairs:0 |
Electron geometry: Trigonal planar Molecular geometry: Trigonal planar Bond Angles: 120 |
|
|
bondings groups: 2 lone pairs:1 |
Electron geometry:Trigonal planar Molecular geometry: bent Bond Angles: < 120 |
|
|
bondings groups:4 lone pairs:0 |
Electron geometry:tetrahedral Molecular geometry: tetrahedral Bond Angles: 109.5 |
|
|
bondings groups: 3 lone pairs: 1 |
Electron geometry: tetrahedral Molecular geometry: trigonal planar Bond Angles: <109.5 |
|
|
bondings groups:2 lone pairs:2 |
Electron geometry: tetrahedral Molecular geometry: bent Bond Angles: <109.5 |
|
|
bondings groups:5 lone pairs:0 |
Electron geometry: trigonal bipyramidal Molecular geometry: trigonal bipyramidal Bond Angles: 120/90 |
|
|
bondings groups:4 lone pairs:1 |
Electron geometry: trigonal bipyramidal Molecular geometry: seesaw Bond Angles:<120 & <90 |
|
|
bondings groups:3 lone pairs:2 |
Electron geometry:trigonal bipyramidal Molecular geometry:t-shaped Bond Angles: <90 |
|
|
bondings groups:2 lone pairs: 3 |
Electron geometry:trigonal bipyramidal Molecular geometry:linear Bond Angles: |
|
|
bondings groups:6 lone pairs:0 |
Electron geometry:octahedral Molecular geometry: octrahedral Bond Angles:90 |
|
|
bondings groups:5 lone pairs:1 |
Electron geometry: octahedral Molecular geometry:square pyramidal Bond Angles: <90 |
|
|
How do you determine the number of valence electrons for any given atom? |
Look at unfilled outermost shell.
Based on group number for main group elements |
|
|
How do you distinguish among ionic bond, non polar covalent bond, polar covalent bond, and metallic bond? |
Electronegativity Difference:
Nonpolar covalent: 0 -.4 Polar covalent: .4 - 2. Ionic- 2--> +
|
|
|
How do you calculate lattice energy for an ionic compound based on use of a Born-Harber Cycle? |
Look at the delta H for each of the following steps:
1. Sublimation of a metal 2. Form an atom from molecule 3. Ionization to form a cation 4. Electron affinity to form anion 5. Find lattice energy |
|
|
How do you predict which compound has the largest lattice energy around a set of ionic compounds? |
Coulomb's Law F= q1 *q2/r
Largest LE has smallest atomic radii, bigger charge makes it larger; Smallest LE has largest atomic radii
Atomic Radius increases going down and decreases across period. |
|
|
What is the difference between electron affinity and electronegativity? |
Electronegativity: The ability of an atom in a molecule to attract electrons to itself. Electron affinity: The energy change for M(g) + e- M-(g). EA deals with isolated atoms in the gas phase. |
|
|
How do you draw Lewis Dot Structures for inorganic and organic molecules and ions? |
Draw atoms, draw valence electrons, form bonds.
*carbon doesn't want lone pairs, Free Radicals- molecules and ions with an odd number of electrons, Incomplete Octets- Boron & Beryllium, Expanded octets-Elements in the 3rd row of the periodic table an beyond often have expand octets up to 12-14 electrons
|
|
|
How do you draw resonance structures for a compound or ion & predict which resonance form is preferred? |
If double or tripple bonds are present, there are probably resonance structures. Look at formal charge, want 0 or negative formal charge. If a positive charge, put it on the most electronegative atom. |
|
|
How do you calculate formal charges for all atoms of a compound or ion? |
Formal charge= Valence - # of pairs- # unpaired |
|
|
What are the exceptions to the octet rule? |
Free Radicals- molecules and ions with an odd number of electrons in their Lewis structures Incomplete Octets-Boron & Beryllium Expanded octets-Elements in the 3rd row of the periodic table an beyond often have expand octets up to 12-14 electrons |
|
|
s orbital |
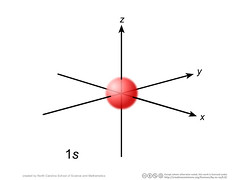
|
|
|
p orbital |

|
|
|
d orbital |
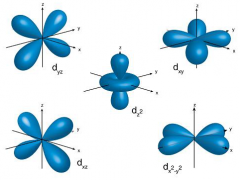
|
|
|
f orbital |
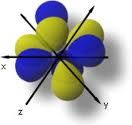
|
|
|
sp hybrid orbital |
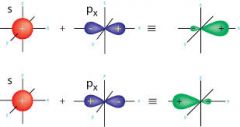
|
|
|
sp3d hybrid orbital |
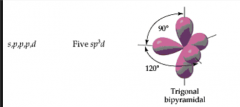
|
|
|
How do you use wedges to show dimensions of molecular geometry? |
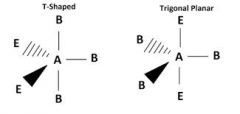
|
|
|
How do you use molecular geometry to determine polarity? |
Lone pairs cause polarity, look at vector sums |
|
|
How do you tell which compound has the largest dipole moment? |
dipole moment= length of bond x charge on each atom
tripple are shorter than double which are shorter than single |
|
|
How do you determine hybridization and bonding schemes in a molecule? |
Same number of bonds |
|
|
How do you identify sigma and pi bonds and which orbital or hybridized orbitals form each bond? |
* pi bond- p orbitals overlap side by side |
|
|
How do you determine whether a molecule has dipole-dipole forces? |
All polar molecules have dipole-dipole forces. |
|
|
How do you interpret an electrostatic potential map of a molecule? |
The redder the area, the more electronegative it is. Size is dependent on atomic radius. |
|
|
How do you determine whether a molecule engages in hydrogen bonding? |
Hydrogen must be bonding to Oxygen, Nitrogen, or Florine. Polar bond. |
|
|
How do you predict relative boiling points of molecules? |
The more cohesive the forces, the higher the boiling point.
ion ion > ion dipole > hydrogen > dipole > dispersion |
|
|
Sublimation |
transition from solid to gas |
|
|
depostition |
gas to solid |
|
|
melting |
solid to liquid |
|
|
freezing |
liquid to solid |
|
|
vaporizing |
liquid to gas |
|
|
condensation |
gas to liquid |
|
|
Miscibility |
Like dissolves like, polar-polar, non-polar-nonpolar |
|
|
Properties of solid |
definite shape, volume |
|
|
Properties of liquid |
definite volume, indefinite shape |
|
|
Properties of a gas |
indefinite shape and volume |
|
|
How do you predict surface tension, viscosity, and volatility? |
Stronger force: more surface tension, more viscous, weaker the force more volatile. |
|
|
Heat of vaporization |
the amount of heat required to vaporize one mole of a liquid to gas |
|
|
heat of fusion |
amount of heat required to melt 1 mole of a solid |
|
|
Dynamic equilibrium |
when the rate of condensation and evaporation become equal |
|
|
Heating curves |
Heating curves aren't curvy. |
|
|
Using heat of vaporization in calcuations |
convert kJ to moles to grams of water |
|
|
Vapor pressure and its dependence on temperature. |
Temperature rises, vapor pressure rises |
|
|
How do you determine whether a solute is soluble in a solvent? |
Like dissolves like |
|
|
Predict whether a molecule would be more soluble in water or in hexane. |
Polar vs nonpolar |
|
|
Entropy increase in solution |
because the potential for randomness is increased |
|
|
What factors improve solubility in water? |
Solids- increase temp Gases & liquids- decrease temp. |
|
|
Enthalpy change upon solution formation: |
Add the enthalpy change for each step 1. Separation of solute particles 2. Separation of solvent particles 3. mixing of the solute and solvent particles (first 2 endothermic, last one exothermic) *in aqueous ionic solution its always exothermic |

