![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
12 Cards in this Set
- Front
- Back
|
Atoms of the same element but having different numbers of neutrons & thus different masses are called....
|
isotopes
|
|
|
Why aren't mass numbers on the periodic table whole numbers?
|
The average of all the masses of the isotopes doesn't calculate to a whole number.
|
|
|
In isotope shorthand, there is a top and a bottom number. Which one identifies the element?
|
The bottom number. It's the number of protons
|
|
|
In isotope shorthand, there is top and a bottom number. What must you do to determine the number of neutrons?
|
Top number minus bottom number
|
|
|
If an isotope has 10 protons and 11 neutrons, then what are the top and bottom numbers in the shorthand?
|
21 on top
10 on bottom |
|

|
Top number says that it has "zero" mass
Bottom number says its charge is -1 |
|

|
Top number says it has a mass of 1 unit.
Bottom number says it has zero charge. |
|
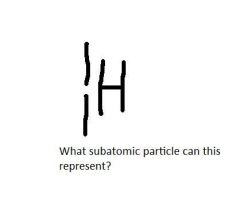
|
Proton
(1-1 = 0 neutrons) |
|
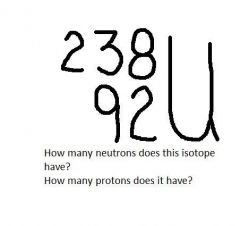
|
146 neutrons (238 - 92 = 146)
92 protons |
|
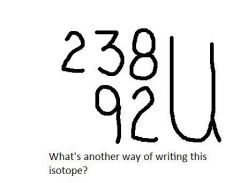
|
U-238
|
|

|
Bottom number 92 identifies it as Uranium.
It has 235 nucleons (143n + 92p = 235) |
|
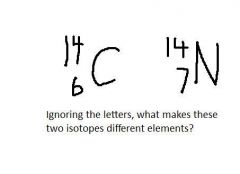
|
Bottom numbers are different (different numbers of protons)
|

