![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
148 Cards in this Set
- Front
- Back
|
Define infectious diseases (ID)
|
1) Disease resulting from tissue destruction or damage which is directly attributable to invasion of the body by a microorganism
2) The study of infectious conditions of the body along with their pathogenesis, associated tissue damage or altered physiology, clinical manifestations, diagnosis, and treatment. |
|
|
Distinguish between principle and opportunistic pathogens
|
Principle pathogen
* Capable of causing infection in normal, healthy individuals - e.g. Staphylococcus aureus Opportunistic pathogen * Does not usually cause infection in healthy individuals * Most likely to cause infection in individuals with compromised host defenses - e.g. Fungal infections caused by Candida, Aspergillus" |
|
|
Define Opportunistic infection
|
Caused by normal flora or transient bacteria when normal host defenses are compromised
* e.g. Candida infections in ICU patients, Pneumocystis pneumonia in AIDS patients * May be caused by either principle or opportunistic pathogens" |
|
|
Define colonization
|
Organism is present but no actual infection occurs
|
|
|
Define Dormant (latent) infection
|
* Asymptomatic carrier state
* Patient is infected, but infection is not progressing and no signs/symptoms - e.g. syphilis, Varicella |
|
|
Define Primary infection
|
Invasion and multiplication of microbes in body tissues causing local tissue injury
* e.g. cellulitis due to S. aureus, UTI's due to E. coli |
|
|
Define Secondary infection
|
Microbial invasion following a primary infection
* e.g. bacterial pneumonia following viral lung infection |
|
|
Define Mixed infection
|
Two or more types of microbes infecting the same tissues
* e.g. abscesses, infections in immunocompromised patients |
|
|
Define Acute infection
|
Rapid onset (hours to days) and brief duration (days to weeks)
* e.g. strep throat |
|
|
Define Chronic infection
|
Prolonged duration (months to years) and slower onset
* e.g. TB, leprosy |
|
|
Define localized infection
|
Confined to a small area or one organ
* e.g. UTI, pneumonia |
|
|
Define generalized infection
|
Disseminated throughout the body
* e.g. Gram-negative bacteremia |
|
|
Define Pyogenic infection
|
Forming pus
* e.g. staphylococcal and streptococcal infections |
|
|
Define Retrograde infection
|
Microbe ascending a duct or tube against the flow of secretions or excretions
* e.g. urinary tract infections |
|
|
Define Fulminant infection
|
Sudden and intense infection
* e.g. meningococcal meningitis |
|
|
Define Community-aquired infection
|
* Infections originate in the outpatient setting
* Patients have had no recent hospitalizations or frequent contact with institutional environment,such as hemodialysis |
|
|
Define Superinfection
|
* Considered a complication or adverse effect of antimicrobial therapy
* Antimicrobial therapy targets pathogenic organisms, but also alters host normal flora * Organisms against which the drug has no activity are then allowed to colonize the host or grow to abnormal populations * Results in new infection with pathogens which are different from those originally treated |
|
|
Define Inapparent (sub-clinical) infection
|
Active infection but with no detectable clinical signs/symptoms
|
|
|
Define Nosocomial (“hospital-associated”)
|
* Infections originate in the hospital or some other institutional setting, e.g. long-term care facility
* Also occur in patients with recent hospitalization or frequent contact with institutional environment |
|
|
The "Holy Trinity" of ID
|
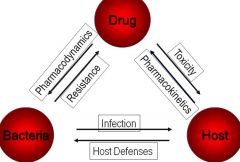
|
|
|
When you are stressed out remember the good times
|

|
|
|
List specific and non-specific host defenses which help to prevent and control infection
|
* Skin and mucous membranes
* Elimination mechanisms - Shedding of epithelial cells, skin cells - Ciliary clearance of airways, cough & sneeze - Urination, lacrimation, peristalsis * Acidity of gastric acids, lacrimal fluids, skin, urine * Enzymes, e.g. lysozymes in tears, bronchial secretions, urine * Gag reflex * Airflow turbulence, humidity in respiratory tract * Cytokines * Fever * Normal Flora |
|
|
What normal flora are associated with the skin?
|
Staphylococcus epidermidis, S. aureus
Propionobacterium acnes |
|
|
What normal flora are associated with the nose and nasopharynx?
|
S. epidermidis, S. aureus
H. influenzae |
|
|
What normal flora are associated with the mouth and tooth surfaces?
|
S. aureus, S. epidermidis,
Streptococcus mitis,& alpha-hemolytic strep Haemophilus Influenzae, Lactobacilus, Bacteroides fragilis, Fusobacterium nucleatum, C. Albicans |
|
|
What normal flora are associated with the large intestine?
|
Escherichia coli, Klebsiella spp., Proteus spp.
B. fragilis, F. nucleatum, Enterococcus, Candida. albicans |
|
|
What normal flora are associated with the vagina and iterine cervix?
|
Bacteroides spp., Clostridium spp.
S. epidermidis, C. albicans, Trichomonas vaginalis |
|
|
Define natural immunity
|
species-specific resistance to diseases of other species
|
|
|
Define passive immunity
|
Antibodis acquired from another source
Vertical - from the mother Artificial - administration of antibodies |
|
|
Define active immunity
|
individual response to exposure to antigen (vaccine)
|
|
|
Role of immunoglobulins
|
- Bind and fix complement
- Opsonization - Neutrophil activation - Cell-independent lysis - Development of specific antibodies - Neutralize toxins - Virus neutralization |
|
|
Examples of Non-specific immunity
|
- Complement
- Fibronectin - Phagocytosis by PMNs and macrophages/monocytes - Acute phase response: a generalized, nonspecific reaction triggered by microbial invasion and host disruption. |
|
|
What host defects can result in infection?
|
* Alteration or suppression of normal flora
* Disruption of natural barriers * Impairment of clearance mechanisms - Respiratory cilia, urine, tears, etc. * Alterations in bowel pH or motility * Immunosuppression - Malnutrition - Underlying diseases - Drugs * Age |
|
|
Define pathogenic organisms
|
Organisms are capable of causing disease
- Principle pathogens - Opportunistic pathogens |
|
|
Define virulence
|
A quantitative measure of pathogenicity, or the potential of an organism to cause disease
|
|
|
Define commensal
|
Organisms in or on the body that do not cause disease
(Usually part of the normal flora) |
|
|
Examples of Non-specific immunity
|
- Complement
- Fibronectin - Phagocytosis by PMNs and macrophages/monocytes - Acute phase response: a generalized, nonspecific reaction triggered by microbial invasion and host disruption. |
|
|
What host defects can result in infection?
|
* Alteration or suppression of normal flora
* Disruption of natural barriers * Impairment of clearance mechanisms - Respiratory cilia, urine, tears, etc. * Alterations in bowel pH or motility * Immunosuppression - Malnutrition - Underlying diseases - Drugs * Age |
|
|
Define pathogenic organisms
|
Organisms are capable of causing disease
- Principle pathogens - Opportunistic pathogens |
|
|
Define virulence
|
A quantitative measure of pathogenicity, or the potential of an organism to cause disease
|
|
|
Define commensal
|
Organisms in or on the body that do not cause disease
(Usually part of the normal flora) |
|
|
Define colonization in the context of the organisms
|
Organisms which are present in or on the body but do not cause clinical illness (Not usually part of the normal flora)
|
|
|
What are the two main sources of infecting microorganisms?
|
* Endogenous:
- Natural flora, commensal organisms - Normally benefit the host but may become pathogenic if translocated * Exogenous: - Acquired from external sources - Carriers: + Humans: Mycobacterium tuberculosis + Animals: Borrelia burgdorferi + Insects: Plasmodium spp. + Objects (fomites): Staphylococcus auerus + Soil: Clostridium tetani + Water: Salmonella typhi + Self |
|
|
Examples of microbial defence mechanisms
|
- Defeat of the complement system
- Avoidance of phagocytosis - Survival inside phagocytic cells - Induction of host immunosuppression - Production of toxins |
|
|
Explain the role of enzyme production in infecting microorganisms
|
* Aid in organism invasion by promoting tissue dysfunction or destruction
* Also involved in destruction of antibiotics: - Coagulase - Catalase - Protease - Hemolysin - Leukocidin - Hyaluronidase - Collagenase - Elastase - β-lactamases - Phospholipases |
|
|
Explain the role of Adherance and Adhesins in infecting microorganisms
|
* Adherence
- Microbial attachment to host cells - First step in host cell killing and toxin delivery - Often provides “tropism” (specific attachment ) - Mediated through adhesins + Filamentous structures (fimbriae or pili) +Other adhesion molecules * Adhesins bind to specific cellular receptors - Galactose - Fibronectin - Lipoteichoic acid - Blood group antigens - Sialic acid - Galactose |
|
|
What are the the main classifications of bacterial toxins?
|
* Exotoxins = proteins actively secreted into surrounding environment or upon bacterial cell lysis
* Endotoxins = component of bacterial membranes, only toxic under certain circumstances |
|
|
Effects of Host on Disease Expression (flow chart)
|
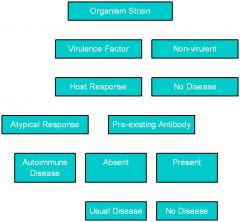
|
|
|
Interaction of Organism with Hosts (flow chart0
|
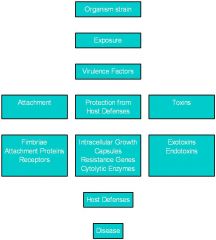
|
|
|
What methods are used to diagnose infectious diseases?
|
* Clinical signs and symptoms * Patient history * Physical examination * Radiological evidence * Gram stain * Culture
|
|
|
List non-specific indicators of infection
|
* Fever * Signs and symptoms * Radiological evidence * Elevated immunoglobulins (non-specific antibodies) * Leukocytosis
|
|
|
Sepsis
|
* Sepsis: clinical evidence of severe infection - A manifestation of more severe infection wich is causing generalized, systemic inflammation and clinical signs and symptoms - Severe sepsis associated with organ system dysfunction, may progress to hemodynamic shock - Septic shock associated with 30 - 50% mortality
|
|
|
List specific indicators of infection
|
* Gram's stain of pathogen * Culture of pathogen * Immunodiagnosis of pathogen - Microbial antigen detection - Antibody detection - Microbial toxin detection
|
|
|
Which organisms commonly cause bacterial bronchitis?
|
* Haemophilus influenzae 24-26%
* Haemophilus parainfluenzae 20% * Streptococcus pneumoniae 15% * Moraxella catarrhalis 15% |
|
|
What are the phases of therapy?
|
1. Empiric (presumptive) 2. Treatment (difinitive or directed treatment)
|
|
|
Phases of Therapy: Empiric
|
* Sometimes referred to as “presumptive” * Usually occurs during the first 72 hours of treatment * Principles: - Identify focus of infection if possible - Assess patient-specific factors which may influence possible pathogens - Collect culture and laboratory tests to help guide later therapy - Select an appropriately BROAD antimicrobial regimen that will optimize control of the infection by covering pathogen(s)
|
|
|
Phases of Therapy: Treatment
|
* Also called “directed” or “definitive” therapy
* Usually occurs during days 3-14 of therapy * Principles: - Utilize culture and susceptibility information to NARROW the spectrum of coverage against the pathogen - Monitor response to therapeutic regimen and possible adverse effects of drugs - Assure compliance with drug therapy - Consider oral or home-based therapy |
|
|
Under what circumstances would Empiric therapy be continued beyond 72 hours?
|
* Suspected infection, but no organism isolated or sample considered contaminated
- Culture sensitivity depends on timing and fluid/tissue cultured * Obtaining samples for culture and microbial identification would be difficult or impractical - Invasive, high risk of complication, high rate of sample contamination |
|
|
Antibiotics Potentially Useful in the Treatment of Bronchitis
|
* Penicillin, amoxicillin
* Amoxicillin/clavulanate * Cefuroxime * Trimethoprim/sulfamethoxazole * Erythromycin, clarithromycin, azithromycin * Levofloxacin, moxifloxacin * Doxycycline |
|
|
Factors to Consider in Antibiotic Selection
|
* Mechanism of action
* Spectrum of activity * Mechanisms and prevalence of resistance * Pharmacokinetic & pharmacodynamic properties * Toxicities and/or adverse effects * Drug interactions * Indications and clinical limitations * Adherence * Cost |
|
|
Define bacteriostatic
|
* Bacteriostatic = arrest growth and replication of organisms; viable organisms may remain & resume growth and replication once antimicrobial is removed from the environment
|
|
|
Define bactericidal
|
* Bactericidal = organisms are killed through the actions of the drug
|
|
|
Define Narrow spectrum of activity
|
Agents act on a single type of organism or relatively limited group of organisms (e.g. only gram-positive bacteria or even only a certain species of bacteria)
|
|
|
Define Broad-spectrum of activity
|
agents act on a wide variety of organisms (e.g. many different gram-positive and gram-negative, aerobic and anaerobic)
|
|
|
T or F: Antimicrobials alone usually cure infections
|
FALSE: antimicrobial agents typically function to limit microbial growth and/or decrease microbial numbers until the host immune system can regain control of the infection This is the idea of suppression vs. eradication.
|
|
|
What host factors influence clinical outcomes in the management of infectious disease?
|
* Genetic determinants * Underlying illness * PK alterations * Age
|
|
|
What drug factors influence clinical outcomes in the management of infectious disease?
|
* MOA * In vitro activity * PK properties * ADEs * duration of therapy
|
|
|
What "bug" factors influence clinical outcomes in the management of infectious disease?
|
* Virulence factors * Intrinsic susceptibility * Resistance mechanisms
|
|
|
Adverse Effects of Penicillins: Hypersensitivity (in general)
|
* Estimated incidence: 1-10%; anaphylactic reactions in ~0.015%
* Half of all allergic drug reactions occur in hospitalized patients receiving high-dose, parenteral agents * Hypersensitivity reactions result from immune system sensitization to chemically reactive breakdown products (e.g. penicilloyl derivatives) - Combine with cellular molecules to form haptens, which function as antigens and initiate antibody production * May be any one of the four types of hypersensitivity reactions |
|
|
Type I reactions to Penicillins
|
* Immediate hypersensitivity leading to anaphylaxis
- Rxn mediated by IgE antibodies * Signs and symptoms: - urticaria - laryngeal edema - bronchospasm with or without hypotension and cardiovascular collapse * Onset 2-20 mins of drug administration |
|
|
Type II reactions to Penicillins
|
* Mediated by IgG and IgM cytotoxic antibodies directed towards penicillin haptens of the cell surface (i.e. RBCs)
* Type II reactions; - hemolytic anemia (Cooms-positive test) - leukopenia - thrombocytopenia - drug-induced nephritis * Usually reversible upon removal of the drug |
|
|
Type III reactions to Penicillins
|
* Not a common rxn clinically
* Occur 1-3 weeks after begining therapy * Caused by circulating antigen-antibody complexes that can deposit in skin, kidneys, blood vessels, and other tissues - Associated with IgG antibodies * Serum sickness syndromes: - rash - fever - arthralgia - lymphadenopathy * Rxns resolve/reverse rapidly after d/c drug |
|
|
Type IV reactions to Penicillins
|
* Delayed rxnx involving lymphocytes and macrophages
* Idiopathic rxns: - pruritis - macropapular rashes - photosensitivity - fixed drug rxn - exfoliative dermatitis - interstitial nephritis * Usually reversible upon drug d/c |
|
|
How are hypersensitivity rxns to Penicillins identified and managed?
|
* Approximately 5-20% of all patients give a history of β-lactam allergy. True allergies is <10%.
* Skin testing - 7-35% of patients with a history of penicillin allergy test positive with a benzylpenicilloyl polylysine skin test - Test not always available, some risk of Type I reaction - Skin testing not usually performed in clinical settings * Best alternative is to treat with an effective non-β-lactam antibiotic if available - PCN desensitization can be performed but often delays initiation of adequate therapy |
|
|
If the presenting patient has a true allergy to Penecillins, what is the risk of cross-reactivity with Cephalosporins?
|
7 - 10%
Risk decreases as the generation of Cephalosporins increases. |
|
|
If the presenting patient has a true allergy to Penecillins, what is the risk of cross-reactivity with Cephalosporins?
|
7 - 10%
Risk decreases as the generation of Cephalosporins increases. |
|
|
If the presenting patient has a true allergy to Penecillins, what is the risk of cross-reactivity with Carbapenems?
|
7 - 10%
|
|
|
If the presenting patient has a true allergy to Penecillins, what is the risk of cross-reactivity with Aztreonam?
|
~0%
|
|
|
If the presenting patient has a true allergy to Cephalosporins, what is the one class of β-Lactam that is contraindicated?
|
Carbapenems
|
|
|
Explain how Clostridium difficile-associated disease
occurs with Penecillin therapy |
* Caused by disruption of normal bowel flora
* Allows overgrowth of Clostridium difficile * C. difficile causes infection of colon - Toxin-mediated inflammation, diarrhea, mucosal injury - Significant morbidity and mortality * Associated with nearly all antibiotic classes, especially broad-spectrum agents with anaerobic activity |
|
|
How does Penecillin therapy lead to supperinfection in some patients?
|
* Antibiotic-induced suppression of susceptible organisms allows growth of different and/or less susceptible organisms
* Leads to new infections with more resistant bacterial organisms as well as fungal organisms, e.g. Candida * Most commonly associated with broad-spectrum antibiotics |
|
|
Penicillins: Drug-Drug Interactions
|
* Aminoglycosides
- Chemical inactivation of penicillin when mixed in same bag, when infused through same IV line, or possibly in patients with severe renal impairment and high, prolonged serum concentrations * Probenecid - Inhibition of renal tubular secretion, increased serum concentration and T1/2 of penicillins |
|
|
What is the benefit of combining β-Lactamase Inhibitors with Penecillins?
|
* Combining penicillins with compounds that specifically and irreversibly inhibit β-lactamases helps restore activity of parent drugs
* β-Lactamase inhibitors usually have no intrinsic antimicrobial activity of their own - Sulbactam is potentially useful exception * Inhibitors irreversibly inhibit β-lactamases via acylation of the enzyme * Not all β-lactamases inhibited by these agents - Effective against penicillinases and β-lactamases produced by many anaerobic organisms - Variable activity against β-lactamases produced by Gram-negative aerobic bacilli |
|
|
Amoxicillin/clavulanate (Augmentin)
|
* Compared to amoxicillin alone, improved activity against S. aureus, many Gram-negative aerobes, and anaerobes
* Available PO only |
|
|
Ampicillin/sulbactam (Unasyn)
|
* Activity similar to amoxicillin/clavulanate, available IV only
|
|
|
Ticarcillin/clavulanate (Timentin)
|
Improved activity against S. aureus, many Gram-negative bacilli including P. aeruginosa, anaerobes
|
|
|
Piperacillin/tazobactam (Zosyn)
|
* Effective against many β-lactamase-producing strains of S. aureus, Gram-negative aerobes (including Pseudomonas), excellent anaerobic activity
* Better overall activity than any other penicillin-class antibiotic * Considered to be a very broad-spectrum agent |
|
|
History of Cephalosporins
|
* 1945 - Fungus (Cephalosporin acremonium) isolated from sea water near a sewage outlet
- Italian professor Guiseppe Brotzu noticed that the water around the outlet was often clear of microorganisms * 1953 - Cephalosporin C successfully isolated from C. acremonium * Several thousand different cephalosporins have been synthesized with goal of identifying new agents with broader spectrum of activity, resistance to b-lactamases * Cephalosporins widely classified by “generation” - Based on antibacterial spectrum of activity * Cephalosporins among the most-used antibiotics clinically, have excellent overall record of efficacy and safety * Cephalosporins are b-lactam antibiotics and have a mechanism of action & resistance similar to the penicillins : Bactericidal |
|
|
Structure of Cephalosporins
|
* Beta-lactam ring attached to Dihydrothiazine ring
* Substitutions at position 3 on the dihydrothiazine ring for differences in metabolism and pharmacokinetics * Substitutions at position 7 alter the antibacterial activity |
|
|
What is so "interesting" about cephamycins?
|
* Several of the second-generation agents are not true cephalosporins
- “cephamycins” - Differ from true cephalosporins in the addition of a methoxy moiety at position 7 - Results in enhanced anaerobic activity, enhanced Gram-negative activity, decreased Gram-positive activity compared to “true” 2nd-generation agents |
|
|
N-methylthiotetrazole (NMTT)
|
* Several cephalosporins were synthesized with a N-methylthiotetrazole (NMTT) moiety at position 3 to increase PBP binding
- Cefamandole - Mandol(IV) - Cefotetan - Cefotan(IV) - Cefmetazole - Zefazone(IV) * Two major undesired effects of NMTT side chain: - Inhibition of Vitamin K epoxide reductase - Inhibition of aldehyde dehydrogenase |
|
|
Discuss the Absorption of Cephalosporins
|
* Absorption highly variable
- Bioavailability 50-70% for most oral agents - Several agents formulated as ester prodrugs to improve absorption |
|
|
Discuss the Distibution of Cephalosporins
|
* Well distributed into most tissues/fluids
- Vd = 0.2 - 0.3 L/kg - CNS levels typically 10-15% of serum, highest with 3rd-generation agents |
|
|
Discuss the Excretion of Cephalosporins
|
* Renal excretion usually ranges from 50 - >90%
- Both filtration and tubular secretion important - Dosing change usually required in renal insufficiency |
|
|
Serum half-life of cephalosporins
|
1-2 hours
|
|
|
What Cephalosporin class drug is active against legionella?
|
None of the Cephalosporins are active against atypical bacteria
|
|
|
What Cephalosporins are active against Pseudomonas aeruginosa?
|
ceftazidime (3rd gen) and cefepime (4th gen)
|
|
|
What Cephalosporins are active against anaerobes?
|
Cephamycins:
Cefoxitin Cefotetan Cefmetazole |
|
|
What Cephalosporins are the most active against gram + aerobes?
|
First Generation
|
|
|
List the First-Generation Cephalosporins
|
* Cephalexin - Keflex (PO)
* Cephradine - Velosef (PO) * Cefadroxil - Duracef (PO) * Cephalothin - Keflin (IV) * Cefazolin - Ancef (IV) * Cephapirin - Cefadyl (IV) |
|
|
Describe the antibiotic activity of the First-Generation Cephalosporins
|
* Relatively narrow in spectrum, primarily focused on Gram-positive activity
- Stable against β-lactamases produced by Gram-positive organisms (e.g. penicillinases), but less stable against those produced by Gram-negative organisms - Limited Gram-negative activity, primarily against enteric bacilli, Moraxella |
|
|
Discuss the Absorption profile of First-Generation Cephalosporins
|
* Acid-stable
* High bioavailability of oral agents (>90%) * Effects of food are variable, but absorption not usually significantly affected (so it is ok to take with or without food) |
|
|
Discuss the Distribution profile of First-Generation Cephalosporins
|
* Good distribution throughout the body
* Minimal CNS penetration |
|
|
Discuss the Elimination profile of First-Generation Cephalosporins
|
* Primarily renally eliminated (>80%)
- Require dosage adjustment in renal impairment |
|
|
What is the half-life of First-Generation Cephalosporins?
|
T1/2 = 0.5-1.6 hours
|
|
|
Spectrum of Activity and Clinical Use of First-Generation Cephalosporins
|
* Primarily focused on Gram-positive activity
- Gram + aerobes: primarily streptococci and staphylococci (except MRSA), no enterococci - Gram - aerobes: Not highly active except certain enteric bacilli (e.g. Klebsiella, E. coli, Enterobacter, Proteus); poor β-lactamase stability - Anaerobes: Limited to PCN-susceptible strains such as those in the oropharynx * Primarily used for skin/soft tissue, bone, and occasionally urinary tract infections |
|
|
List the Second-Generation Cephalosporins
|
* Cefaclor - Ceclor (PO)
* Loracarbef - Lorabid (PO) * Cefprozil - Cefzil (PO) * Cefuroxime axetil - Ceftin (PO) * Cefuroxime - Zinacef (IV) * Cefamandole - Mandol (IV) (NMTT) * Cefonicid - Monocid (IV) Cephamycins: * Cefoxitin - Mefoxin (IV) * Cefotetan* - Cefotan (IV) * Cefmetazole* - Zefazone (IV) |
|
|
Discuss the Absorption profile of Second-Generation Cephalosporins
|
* Acid-stable with overall good bioavailability of oral agents
* Cefuroxime axetil should be administered with food, otherwise absorption of other agents not usually significantly affected by food |
|
|
Discuss the Distribution profile of Second-Generation Cephalosporins
|
* Good distribution throughout the body
- Only cefuroxime has decent CNS penetration, but not as good as third-generation agents |
|
|
Discuss the Elimination profile of Second-Generation Cephalosporins
|
* Primarily renally eliminated (50 - >90%)
- Require dosage adjustment in renal impairment |
|
|
What is the half-life of Second-Generation Cephalosporins
|
T1/2 = 1 - 4.5 hours
|
|
|
Spectrum of Activity and Clinical Use of Second-Generation Cephalosporins
|
* Broader in spectrum, improved Gram-negative activity compared to first-generation cephalosporins
- Gram + aerobes: Primarily streptococci and staphylococci, not quite as active as first-gen. agents - Gram - aerobes: Improved Gram-negative activity against enteric bacilli (e.g. E. coli, Klebsiella, Enterobacter, Proteus) - Also good activity against some selected β-lactamase producing organisms, e.g. Haemophilus influenzae, Moraxella catarrhalis - Anaerobes: Limited overall + Exception: cephamycins have best activity of cephalosporin class, including Bacteroides fragilis * Various agents used for skin/soft tissue, respiratory tract, intra-abdominal, and other infections; useful for mostly community-acquired infections |
|
|
List the Third-Generation Cephalosporins
|
* Cefixime - Suprax (PO)
* Cefpodoxime proxetil - Vantin (PO) * Cefdinir - Omnicef (PO) * Ceftibuten - Cedax (PO) * Cefditoren pivoxil - Spectracef (PO) * Cefotaxime - Claforan (IV) * Ceftizoxime - Cefizox (IV) * Ceftriaxone - Rocephin (IV) * Cefoperazone* - Cefibid (IV) (NMTT) * Ceftazidime - Fortaz (IV) |
|
|
Describe the antibiotic activity of Third-Generation Cephalosporins
|
* Broad spectrum agents with excellent Gram-negative activity
- Much more stable against many β-lactamases produced by Gram-negative organisms as well as those produced by Gram-positive organisms (e.g. penicillinases) |
|
|
Discuss the Absorption profile of Third-Generation Cephalosporins
|
* Good bioavailability of oral agents
* Cefpodoxime and cefditoren esters much better absorbed when given with food - Give cefditoren with a high-fat meal |
|
|
Discuss the Distribution profile of Third-Generation Cephalosporins
|
* Good distribution throughout the body
* CNS penetration of IV agents reasonably good, makes these agents useful for CNS infections |
|
|
Discuss the Elimination profile of the Third-Generation Cephalosporins
|
* Most agents primarily renally eliminated (50->90%)
- Most agents require dosage adjustment in renal impairment * Ceftriaxone and cefoperazone only 30-45% renally eliminated, extensively eliminated in bile - Do not require dosage adjustments in renal dysfunction |
|
|
Half-life of Third-Generation Cephalosporins
|
* Most agents T1/2 = 2-4 hours
* Ceftriaxone T1/2 = 8 hours |
|
|
Spectrum of Activity and Clinical Use of Third-Generation Cephalosporins
|
* Useful for broad range of community-acquired and nosocomial infections (IV agents) due to good Gram-positive and excellent Gram-negative activity
- Gram + aerobes: primarily streptococci and staphylococci, less active than other generations but still clinically useful - Gram - aerobes: Active against wide range of organisms, but still subject to inactivation by many β-lactamases + Excellent against Enterobacteriaceae +Also excellent against H. influenzae, M. catarrhalis, Neisseria - Ceftazidime has good activity against P. aeruginosa - Anaerobes: Limited to oropharyngeal strains * Used for wide range of infections in most organ systems/tissues, including CNS infections and serious Gram-negative infections in hospitalized patients |
|
|
List the Fourth-Generation Cephalosporins
|
Cefepime – Maxipime (IV)
|
|
|
Discuss the antibiotic activity of the Fourth-Generation Cephalosporins
|
* Has excellent Gram-negative activity, including P. aeruginosa
- More stable than third-generation agents to many β-lactamases, particularly those produced by Enterobacteriaceae |
|
|
Discuss the Distribution profile of Fourth-Generation Cephalosporins
|
Good CNS penetration
|
|
|
Discuss the Elimination profile of the Fourth-Generation Cephalosporins
|
renally eliminated (85%)
|
|
|
What is the half-life of the Fourth-Generation Cephalosporins?
|
T1/2 = 2 hours
|
|
|
Clinical Use of Fourth-Generation Cephalosporins
|
Useful in treating wide range of serious infections, similar to third-generation agents but often less resistance
|
|
|
Ceftaroline
|
* Not yet clinically available, but potential for FDA approval in 2011
* Similar to ceftriaxone in most respects, i.e. good Gram-negative activity * Also greatly enhanced affinity for PBP2a * Ceftaroline will be the first -lactam to have clinically useful activity against methicillin-resistant S. aureus (MRSA) and Enterococcus * Very unique spectrum of activity among the cephalosporins |
|
|
Cephalosporins: Adverse Effects
|
* Cephalosporins are considered to be among the safest of antimicrobial and are associated with few serious adverse effects
* Side effect profile generally similar to penicillins * Hypersensitivity reactions occur in 1-3% of patients - Reactions range from rash to anaphylactic reactions - Approximately 7-10% of patients with true penicillin allergy will have cross-sensitivity to cephalosporins - Many patients will develop rash to penicillins (e.g. ampicillin), does not necessarily contraindicate cephalosporin administration but care should be exercised * Ceftriaxone associated with biliary sludging * Agents with NMTT side chain: - Hypoprothrombinemia, increased bleeding times - Disulfiram-like reactions when used in patients with recent alcohol use * C. difficile-associated disease, superinfection |
|
|
Cephalosporins: Drug-Drug Interactions
|
* Probenecid
- Inhibition of renal tubular secretion, increased serum concentration and T1/2 of cephalosporins * Antacids, H2-receptor antagonists, PPIs +Significantly decrease bioavailability of agents administered as ester prodrugs (cefuroxime axetil, cefpodoxime proxetil, cefditoren pivoxil) and also cefdinir * Ferrous sulfate (& other iron products) - Significantly decreased absorption of cefdinir * Alcohol - Agents with NMTT side chain |
|
|
Structure of Carbapenems
|
* Semisynthetic Beta-lactam antibiotics which are structurally similar to penicillins
* Differ from penicillins in two main features: - Replacement of sulfur atom at position 1 with a carbon - Unsaturated bond between positions 2 * Carbapenem nucleus highly resistant to hydrolysis by common β-lactamase enzymes - Makes the carbapenems very active against many organisms which are resistant to penicillins and cephalosporins * Imipenem extensively hydrolyzed and inactivated by human renal dehydropeptidase I (DHP-I) - Enzyme found in brush border of proximal renal tubular cells - Cilastatin inhibits DHP-I, is co-administered with imipenem in a 1:1 ratio to make imipenem more clinically useful * Meropenem, ertapenem, and doripenem have methyl group at the 1 position of the nucleus - Confers resistance to DHP-I hydrolysis, co-administration with inhibitor agent not necessary * Differences in the side chain at the 2 position of the nucleus account for differences in antibacterial activity among various agents |
|
|
Spectrum of Activity of the Carbapenems
|
* Carbapenems exhibit the broadest antibacterial activity of any β-lactam antibiotics – or any other class!
* Excellent activity against aerobic Gram-positive organisms - Active against most staphylococci and streptococci - Moderate (not great) activity against Enterococcus faecalis - No activity against MRSA, Enterococcus faecium * Outstanding activity against aerobic Gram-negatives - Enterobacteriaceae, Haemophilus, Moraxella, Neisseria - Imipenem, meropenem, and doripenem have excellent activity against most non-fermenting organisms (P. aeruginosa, Acinetobacter) but not Stenotrophomonas maltophila - Ertapenem much less active against non-fermenters but otherwise similar to other agents * Excellent activity against clinically important anaerobes |
|
|
MOA of Carbapenems
|
Mechanism of action similar to other β-lactam antibiotics - Bactericidal
|
|
|
Mechanisms of Resistance for the Carbapenems
|
* Mechanisms of resistance are also similar to other β-lactams, but a couple of differences
- Less resistance related to β-lactamase enzymes - Carbapenemase enzymes (i.e. KPCs) are relatively infrequent but are becoming more common in some geographic areas - Porin modifications, decreased penetration a major mechanism of resistance for the carbapenems - Efflux also becoming a more widely recognized & important mechanism, especially in combination with porin alterations |
|
|
Discuss the Absorption profile of Carbapenems
|
* Poor oral absorption
- No oral agents currently available in U.S. (under investigation) - Oral agents available in other parts of the world |
|
|
Discuss the Distribution profile of Carbapenems
|
* Well distributed throughout the body
* Good CNS penetration, clinically useful for CNS infections |
|
|
Discuss the Elimination profile of Carbapenems
|
* Renally eliminated (>70 – 80%)
- Dosage adjustments required in patients with renal impairment |
|
|
Half-life of Carbapenems
|
* Imipenem, meropenem, doripenem = 1 hour, require Q6-8H dosing
* Ertapenem = 4 hours, allows for QD dosing |
|
|
Carbapenems: Adverse Effects
|
* Generally well tolerated, adverse effect profile similar overall to penicillins and cephalosporins
* Hypersensitivity reactions - Considerations very similar to those for cephalosporin use - Approximately 7-10% of patients with true penicillin allergy will have cross-sensitivity to cephalosporins - Cross-sensitivity of carbapenems to cephalosporins considered to be 100% * Neurotoxicity - Unique to carbapenems among β-lactam agents - Mechanism thought to be related to inhibition of GABA receptors - Dizziness, headache, insomnia in 2 - 8%, seizures in 0.1 – 1% - Seizures most common with imipenem - Risk factors include impaired renal function, failure to properly adjust doses for renal impairment, elderly, other CNS conditions (e.g. trauma, CVA, infection, tumor) * C. difficile-associated disease, superinfection |
|
|
Carbapenems: Drug-Drug Interactions
|
* Probenecid - Inhibition of renal tubular secretion, increased serum concentration and T1/2 of carbapenems
* Valproic acid - Increased hepatic metabolism of VPA, potential for decreased concentrations of VPA and loss of seizure control |
|
|
Carbapenems: Clinical Use
|
* Very widely used (and overused) due to their very broad spectrum of activity
* Clinically useful in wide variety of severe infections in hospitalized patients - Respiratory tract, skin/soft tissue, intra-abdominal, bloodstream, CNS, gynecologic, others * Particularly useful in treatment of highly resistant organisms for which other antibacterial agents are not effective - Valuable for treatment of nosocomial infections due to risk of infection with multidrug-resistant pathogens |
|
|
Structure of the Monobactams
|
* Aztreonam - Azactam (IV)
* Semisynthetic β-lactam antibiotic which is structurally unique - Has only the four-member β-lactam ring as its central nucleus - Methyl group at 4 position confers stability against β-lactamases - Side chain confers Gram-negative activity |
|
|
Spectrum of Activity of Aztreonam
|
* Mechanisms of action & resistance similar to other Beta-lactam antibiotics
- Beta-lactamases, decreased penetration of membrane most important for aztreonam * Excellent activity against aerobic Gram-negative bacilli - Includes good activity against P. aeruginosa - Overall, Gram-negative activity very similar to ceftazidime * Aztreonam possesses NO clinically useful Gram-positive or anaerobic activity |
|
|
Discuss the Absorption of Aztreonam
|
Poor oral absorption
|
|
|
Discuss the Distribution profile of Aztreonam
|
* Well distributed throughout the body
* CNS penetration similar to penicillins, inferior to third-generation cephalosporins and carbapenems |
|
|
Discuss the Elimination profile of Aztreonam
|
* Renally eliminated (60 – 70%)
- Dosage adjustments required in patients with renal impairment |
|
|
Half-life of Aztreonam
|
T1/2 = 1.7 – 2.0 hours
|
|
|
Aztreonam: Adverse Effects
|
* Generally well tolerated, adverse effect profile similar overall to other Beta-lactams
* Hypersensitivity reactions - Cross-sensitivity to other Beta-lactam agents is rare (<<1%) - May be considered an option in patients with severe allergy to other Beta-lactam antibiotics - Exception may be in patients who are allergic specifically to ceftazidime because side chains are nearly identical and immunogenic * C. difficile-associated disease, superinfection |
|
|
Aztreonam: Drug Interactions
|
No important drug-drug interactions
|
|
|
Aztreonam: Clinical Use
|
* Clinically useful in wide variety of severe infections in hospitalized patients
- Respiratory tract, skin/soft tissue, intra-abdominal, bloodstream * Current use is mostly limited to patients with Gram-negative infections and severe allergy to other Beta-lactam antibiotics |

