![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
17 Cards in this Set
- Front
- Back
|
What is an ionic compound? |
A compound that contains positive and negative ions held together in a regular arrangement (lattice) by electrostatic forces of attraction |
|
|
What is an ionic lattice? |
A closely-packed regular arrangement of particles held together by electrostatic forces of attraction |
|
|
What is a lattice? |
A closely-packed regular arrangment of particles |
|
|
What is an electrostatic force? |
A force of attraction between opposite charges |
|
|
Advantage of Dot and Cross diagrams |
Useful for showing how ionic compounds are formed |
|
|
Disadvantage of Dot and Cross diagrams |
Don't show the structure of the compound, the relative sizes of the ions or how they're arranged |
|
|
Advantages of 3D Models |
Show the relative sizes of the ions, as well as the regular pattern in an ionic crystal |
|
|
Disadvantages of 3D Models |
They only let you see the outer layer of the compound |
|
|
Advantages of Ball and Stick Models |
Show the regular pattern in an ionic lattice, as well as how all the ions are arranged May show the relative sizes of the ions |
|
|
Disadvantages of Ball and Stick Models |
Sometimes the ions are not shown to scale They suggest that there are gaps between the ions, when in reality there aren't. |
|
|
Working out the formula of an ionic compound from a Dot and Cross diagram |

|
|
|
Properties of ionic compounds Solubility |
Most ionic compounds dissolve easily in water |
|
|
Working out the formula from a Ball and Stick Model |

|
|
|
Working out the formula of an ionic compund from 3D Models |
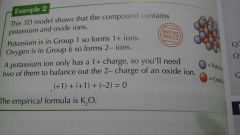
|
|
|
Properties od ionic compounds. Melting and Boiling points. |
Ionic compounds all have high melting and boiling points due to the strong electrostatic attraction between ions. It takes a large amount of energy to overcome this attraction and break the many bonds |
|
|
Properties of ionic compounds Electrical conductivity |

|
|
|
What is an emperical formula? |
A chemical formula showing the simplest possible whole number ratio of atoms in a compound |

