![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
54 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Type of bond between a metal and a nonmetal is.... |
Ionic bond
|
|
|
|
How can you quickly find the number of valence electrons in a atom?
|
Look at the group number.
|
|
|
|
When atoms share electrons, what kind of bond is created?
|
Covalent bond
|
|
|
|
In an ionic bond, nonmetals tend to ______ electron(s).
|
gain
|
|
|
|
In an ionic bond, metals tend to _____ electron(s).
|
lose
|
|
|
|
When metals lose electrons, they tend to have the configuration of the noble gas that ______ them.
|
precedes
|
|
|
|
When nonmetals gain electrons, they tend to have the configuration of the noble gas that _____ them.
|
follows
|
|
|
|
What type of oxidatioon number is formed when metal atoms become ions?
|
positive; called cations
|
|
|
|
What type of oxidation number is formed when nonmetal atoms become ions?
|
negative; called anions
|
|
|
|
How many dots do the dot structures of the alkali metals have?
|
one
|
|
|
|
How many dots do the dot structures of the alkaline earth metals have?
|
two
|
|
|
|
How many dots do the dot structures of the halogens have?
|
seven
|
|
|
|
How many dots do the dot structures of the noble gases have?
|
eight, except for helium, which has two
|
|
|
|
How many dots do the dot structures of group VI A have?
|
six
|
|
|
|
How many dots do the dot structures of group VA have?
|
five
|
|
|
|
How many dots do the dot structures of group IV A have?
|
four
|
|
|
|
How many dots do the dot structures of group III A have?
|
three
|
|
|
|
Because of the d electrons of the transtion metals, what is typical of oxidation numbers in B elements?
|
They have multiple oxidation numbers, typically 2+ and another one or two
|
|
|
|
What oxidation numbers do Zinc and Cadmium always have, due to their location in the d-block?
|
2+
|
|
|
|
What oxidation number does Silver always have due to its location in the d-block?
|
1+
|
|
|
|
Do groups I A and II A ever have multiple oxidation numbers?
|
No
|
|
|
|
When an element has multiple oxidation numbers, how do you show it in a formula's name?
|
Use a Roman Numeral to show which positive charge it has.
|
|
|
|
What are the names of Fe^2+, Fe^3+, and Fe^6+?
|
Iron II ion
Iron III ion Iron VI ion |
|
|
|
Which Noble Gas does Rubidium resemble, when it loses one electron?
|
Krypton
|
|
|
|
Which Noble Gas doe Bromine resemble when it gains one electron?
|
Krypton
|
|
|
|
When halogen elements become ions what do we call those anions?
|
halides
|
|
|
|
What is the name of the ion made from chlorine?
|
chloride ion
|
|
|
|
What is the name of the ion made from fluorine?
|
fluoride ion
|
|
|
|
What is the name of the ion made from iodine?
|
iodide ion
|
|
|
|
What is the name of the ion made from bromine?
|
bromide ion
|
|
|
|
What is the name of the anion made from oxygen?
|
oxide ion
|
|
|
|
What is the name of the anion made from sulfur?
|
sulfide ion
|
|
|
|
What is the name of the anion made from nitrogen?
|
nitride ion
|
|
|
|
What is the name of the anion made from selenium?
|
selenide ion
|
|
|
|
What is the name of the anion made from carbon, (which is rare)?
|
carbide
|
|
|
|
When single nonmetal element becomes an anion, it carries the _____ suffix.
|
-ide
|
|
|
|
When a nonmetal element is combined with oxygen in an anion, it usually carries the _____ suffix.
|
-ate
|
|
|
|
Ions of only one element are called _____ ions.
|
Monoatomic
|
|
|
|
Ions of more than one element are called ____ ions.
|
Polyatomic
|
|
|
|
When an ion has 2 elements in it, the second element is usually _____.
|
oxygen
|
|
|
|
How would you determine the formula for Iron III oxide
|
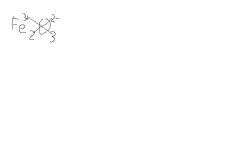
|
|
|
|
How would you determine the formula for Iron II oxide?
|
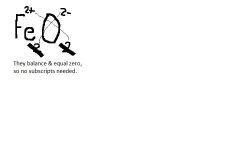
|
|
|
|
What is the name of CuS?
|

Since the S is 2-, and there are no subscripts, then the Cu must be 2+
|
It must have a Roman Numeral since Cu is a transition metal?
|
|
|
Which common transition metals never have more than one oxidation number & thus don't need Roman Numerals when naming them?
|
Silver (Ag)
Cadmium (Cd) Zinc (Zn) |
|
|
|
What happens to ionic compounds when hit with a hammer?
|
They shatter because they are hard & brittle.
|
|
|
|
Ionic compounds are typically ______ solids.
|
Crystalline
|
|
|
|
Dry Ionic compounds are typically _____-condutors of electricity.
|
Non
|
|
|
|
When ionic compounds are dissolved in water or are melted, they become ______ electrical condutors.
|
Excellent
|
|
|
|
What is the chemical formula for Potassium oxide?
|

|
Potassium, K is 1+
Oxygen, O is 2- |
|
|
Ionic compounds tend to have a______ melting point temperature.
|
High
|
|
|
|
The ____ of electrons typical of metals is what causes malleability, conductivity, and luster.
|
sea
|
|
|
|
Blends of metals together are called...
|
alloys
|
|
|
|
Bonds holding metal atoms together are called.....
|
metallic bonds
|
|
|
|
Why isn't MgO called Magnesium II oxide?
|
Magnesium is NOT a transition metal, & thus doesn't need a Roman Numeral.
|
|

