![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
61 Cards in this Set
- Front
- Back
|
how energy of a photon relate to freq and wavelength |
*the shorter the wavelength the more energy there is *v=1/wavelength *the freq of a wave is inversely proportional to wavelength *E=hv (h is planks constant) |
|
|
%T |
A=2-log%T |
|
|
considering beers law how does %T, A and C change with width of cuvette |
inc width *%T dec *A inc c dec
dec width %T inc A dec C inc |
|
|
Beers law conditions |
1. incident radiation is monochromatic 2. solvent absorption is insignificant 3. solute conc is w/in given limits 4. optical interferent isn't present 5. chem. rxn doesn't occur btwn the molecules of interest and another solute or solvent molecule |
|
|
100% T |
0 absorbance *you want to pick a wavelength that is at max abs! (0 %T) |
|
|
why a soln that appears blue is measured with a wavelength that appears yellow |
*absorbing everything but blue (transmitting blue) *perceived color of a soln will be the wavelengths that aren't absorbed by solute *complementary color is what has max absorbance |
|
|
basic spec (in order) |
1. light source 2. entrance slit 3. monochromator 4. exit slit 5. cuvette 6. detector 7. meter |
|
|
sources of radiant energy (light source) |
*tungsten filament *deuterium discharge *mercury vapor *lasers *mercury arc *xenon flash |
|
|
types of monochromators (starting w/ least efficient) |
1. glass or plastic filters 2. interference filters 3. multiple interference filters 4. prisms 5. diffraction grating |
|
|
type of detectors (starting with most simple) |
1. photovoltaic cells (barrier layer cell) 2. phototubes 3. photomultiplier tube 4. photodiode detector |
|
|
used to check linearity of detector response |
*NDDS *solid glass filters *solns of varying [ ] of a cmpd known to follow beers law and plot on graph to check |
|
|
how to check for stray light |
cutoff filters |
|
|
consequence of stray light |
*cause errors in the high abs range *at high analyte conc it dec. in absorbance |
|
|
check for wavelength accuracy |
*didymium or holmium oxide in glass placed in light path and wavelength control is set where the abs max is expected *some use Hg-vapor lamp w/ sharp known emission lines |
|
|
how do photosensitive detectors work |
*converts radiant energy to electrical energy by light sensitive surface that release e in #'s proportional to the intensity of the light striking it |
|
|
calc. bilirubin conc if 10 mg/dl std reads 0.30 |
(A1/C1)=(A2/C2) solve for C2 |
|
|
principle of atomic absorbtion |
*detects absorption of EMR by atoms rather than molecules *voltage ionizes the filler gas and ion are attracted to metal cathode that knock atoms off and cause the metal atoms to be excited. *as they return to ground state light energy is emitted *analyte atoms remain in ground state and absorb light energy *light source=hollow cathode lamp *flame=to form free, unexcited atoms |
|
|
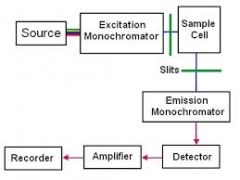
principle of molecular fluorescence |
*beginning is similar to basic spectrophotometry *though it has 2 monochromators to select the excitation and emission wavelengths *detector is at a right angle to excitation beam *fluorescent cmpds are most often compds w/ alternating double bonds |
|
|
diagram of molecular fluorescence |
|
|
|
excitor wavelength |
*high energy, high freq, and short wavelength
|
|
|
emission wavelength |
*longer wavelength, less energy, low freq --takes energy to make fluorescent emission |
|
|
ex of specific types of potentiometric ISE electrodes |
*Na *K *Cl *Li *H (pH electrode) *result depends on the log of ion activity |
|
|
antibiotic that has a high structural affinity for potassium |
valinomycin |
|
|
electrode based on principle of amperometry |
PO2 *AKA Clark Oxygen electrode |
|
|
mode |
value in sample that occurs w/in greatest freq |
|
|
range |
difference btwn the highest and lowest values in the sample |
|
|
standard deviation |
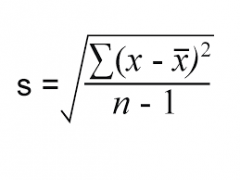
|
|
|
coefficient of variation |
CV%=S/x bar (100) *same thing as relative std deviation |
|
|
standard deviation interval |
describes how many std. deviations a particular value is above or below the mean *lab value-group mean/group SD |
|
|
standard |
a soln that contains a known amount of analyte and is used to calibrate a method |
|
|
control |
used to monitor the performance of a method after it has been calibrated |
|
|
what conditions are screened for in prenatal risk screenings |
*open neural tube defects (spina bifida, anacephaly) *down syndrome/ trisomy 21 *trisomy 18 *tests maternal serum 1 if + then test amniotic fluid |
|
|
tests included in prenatal risk screening |
*alpha fetoprotein *unconjugated estriol (UE3) *human chorionic gonadotrophin *dimeric inhibin A |
|
|
alpha fetoprotein |
inc in open neural tube defects |
|
|
unconjugated estriol |
dec in downs and trisomy 18 |
|
|
human chorionic gonadotrophin |
inc in downs dec in trisomy 18 |
|
|
dimeric inhibin A |
inc in downs |
|
|
MoM |
Multiple of the Median *risk factors are based on MoM for ea. analyte. There's a database of median values for ea. analyte for ea. gestational week |
|
|
working principles of AU480 |
*spectrophotometer (including enzymes) *ISE Na, K, Cl *iCal *osmometer *extra ( NADH absorbs max at 340) |
|
|
working principle of NOVA8 |
*ionized ca is measured by electrochemical means *measuring electrodes -Na -K -Mg -Ca *reference electrode *pH electrode (H) *calc. Hct and normalized Ca |
|
|
relationship btwn ionized ca and pH |
*Inversely w/ pH *alkalosis favors association which dec Cai *why run: premature babies (not enough albumin), transfusions |
|
|
preferred acceptable spec for whole blood ionized ca from a neonate |
*collected using a balanced 'preheparinized' syringe *sent to lab in ice slurry to maintain pH |
|
|
how quickly should whole blood ionized ca be analyzed |
w/ in 30 min |
|
|
unacceptable samples for ionized ca |
*hemolyzed spec *frozen samples on sep gel *sample aliquots *spec shipped on dry ice *frozen whole blood *citrate, EDTA, Oxalate fl tubes (chelates Ca) |
|
|
tetany |
substantial dec. in ionized ca resulting in a state of neuromuscular excitability |
|
|
ref interval for total and ionized ca age 19+ |
4.75-5.30 mg/dl (ionized) 8.5-10.5 mg/dl (total) |
|
|
how often are controls run on NOVA 8 |
every 8 hrs |
|
|
established linearity for ionized ca |
2.2-8.5 mg/dl |
|
|
enzyme that oxidizes the oxalate in the urine oxalate procedure |
oxalate oxidase |
|
|
manufacturer's stated stability of the oxalate controls |
2 days refrigerated (at SH they freeze it and it lasts up to 4 wks) |
|
|
principle of freezing point depression |
*supercool soln several deg below its freezing point *raise temp by heat fusion up to soln freezing pt and then plateaus *calculate result off of plateau (*vapor pressure depression is another method you can use) |
|
|
Principle solute components that contribute to serum osmotic conc |
*Na, Cl, glucose, urea, alcohol, etc |
|
|
ref interval for urine osmolality |
50-1200 mOsm/kg |
|
|
freq osmometer controls run |
every 8 hrs |
|
|
how often is the 290 mOsm/kg std run |
daily |
|
|
what are 3 clinical conditions where osmolality measurements are useful |
1. dehydration (ADH issues) 2. detection of hyper/hyponatremia, false hyponatremia, hyperglycemica 3. blood alcohol |
|
|
light source |
to produce desired wavelength |
|
|
entrance slit |
to minimize light and focus light on monochromator |
|
|
monochromator |
isolates wavelength |
|
|
exit slit |
focuses monochromatic light |
|
|
detector |
converts transmitted radiant energy into electrical energy |

