![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
192 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
In order for infection to occur what barrier does the infectious organism initially have to pass? What mediates protection at these surfaces?
|

To enter the body, an infectious organism initially has to pass the external defences which include the physical barrier of the skin and internal epithelial layers lining the major tracts of the body. Protection at these surfaces is mediated by a variety of secretions.
|
|
|
|
What is the general difference between innate and adaptive immunity?
|
INNATE IMMUNE SYSTEM:
>First line of defence against infection >Works rapidly but has no memory >Gives rise to the acute inflammatory response >Does not vary response depending on infectious agent >Has broad specificity ADAPTIVE/ACQUIRED IMMUNITY: >Takes longer to develop >Highly specific >Responds more quickly to a microbe that it has encountered previously (i.e. has memory for antigen) |
|
|
|
Describe the three levels of human immune defence.
|
EXTERNAL DEFENCES
- if breached the organism encounters: INNATE IMMUNE RESPONSE - if necessary support recruited from: ADAPTIVE IMMUNE RESPONSE |
|
|
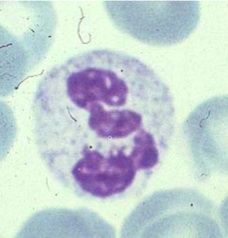
Identify this cell and its function.
|
>Neutrophil (polymorphonuclear)
>Found in blood and the tissues >Phagocytic cell >Innate immune system |
|
|
|
Draw the haematopoietic differentiation pathway, highlighting the lymphoid and developmental pathways.
|
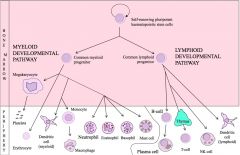
|
>FDCs are NOT derived via haematopoiesis.
>Dendritic cells can be derived from both myeloid/lymphoid paths. >Erythrocytes and platelets also play an important role in the immune response. |
|
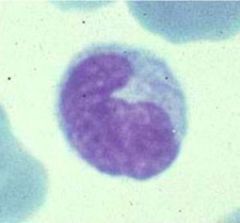
Identify this cell type and its function.
|
>Monocyte (in blood) / Macrophage (in tissues, differentiates upon leaving the blood)
>Developed via myeloid pathway >Phagocytic >Cell of innate defence > |
|
|
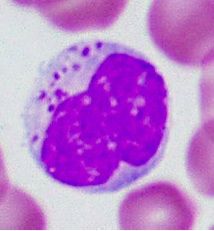
Identify this cell and its function.
|
>Natural killer cell
>Present in blood and tissues >Kills cells infected with virus (non phagocytic) |
|
|
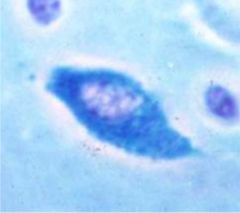
Identify this cell and its function.
|
>Mast cell (granulocyte)
>Found on mucosal surfaces and in connective tissues >Promotes inflammation via release of inflammatory mediators IL8 and PG >Cell of innate immunity |
|
|
|
What are the 5 main classes of molecule of the innate immune system?
|
1. Complement proteins:
>20-30 proteins - cascade system >Play a role in phagocytosis, lysis and inflammation 2. Acute phase proteins: >Plasma concentration may increase 1000x during infection >Produced in liver - C-Reactive Protein (CRP): Activates complement and used as a marker - Serum amyloid P component: Amyloid precursor - Mannose-binding lectin (MBL or MBP, protein that binds carbohydrate): activates complement 3. Cytokines e.g. interferons α, β, and γ 4. Defensins >Small cationic antimicrobial peptides - widely expressed by epithelia and leukocytes 5. Pattern recognition receptors (PRR) - Toll-like receptors - Recognise PAMPs e.g. LPS |
|
|
|
What is the role of the acute phase response?
|
>Enhances host resistance
>Minimises tissue injury >Promotes resolution and repair of the inflammatory lesion |
|
|
|
What types are there and what role does interferon play?
|
>IFN α, β, and γ
>Inhibit virus replication >Increase activity of NK cells (which kill virus) >Increase expression of MHC molecules involved in presentation of antigens to T cells >IFN γ is produced by Th cells (link with adaptive immunity) and by NK cells |
|
|
|
How do innate immune cells recognise microbes differently from lymphocytes?
|
>Cells of the innate response have pattern recognition receptors (PRR e.g. Toll-like receptors) to recognise microbial pathogen associated molecular patterns (PAMPs) e.g. Mannose, LPS, microbial nucleic acids.
>Lymphocytes of the adaptive response have TCR or BCR to recognise components of the pathogen - antigenic epitopes. |
|
|
|
Considering the cells (their function and location), specificity, molecules, speed and duration and memory compare the innate vs. adaptive immune systems.
|
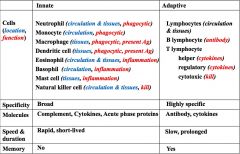
|
|
|
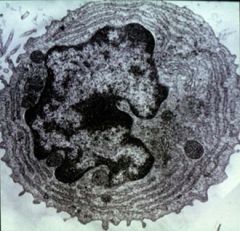
Identify this cell and its function.
|
>Plasma cell (B lymphocyte)
>Develops antibodies and secretes them >Supported by T helper cells |
|
|
|
Describe the differences and similarities between T cells and B cells.
|

|
|
|
|
Provide examples of hypersensitivity disease and the corresponding antigens.
|

|
|
|
|
Describe the role of the different forms of T cells.
|
>Th cells help:
- B cells produce antibody - Macrophages to become activated - Cytotoxic T-cells to become activated >Regulatory T cells (Treg): - Suppress excessive immune responses >Cytotoxic T-cells (CTLs) - Kill infected cells |
|
|
|
What is adaptive immunity based on?
|
Entirely on properties of lymphocytes:
1. Highly specific: - responses tailored to exact antigen that induced them 2. Start slowly: - often days before a response occurs, then prolonged increasing response (due to proliferative powers of lymphocytes) 3. Development of immunological memory: - Ensures subsequent encounter with the same antigen is faster and more effective - Based on production of memory cells - Vaccination works on this principle |
|
|
|
Where are lymphocytes predominantly found?
|
Lymphoid tissues and organs (Thymus, Bone marrow, Spleen, Lymph nodes, MALT)
|
|
|
|
Describe what is meant by the complement system.
|
>The complement system involves a series of molecules, including enzymes, which act in a cascade fashion to generate components actively involved in host defence mechanisms.
>Can be activated in 3 ways: - Classical pathway (antigen antibody complexes) - Lectin pathway (MBL) - Alternative pathway (microbial components e.g. cell wall) >It 'complements' the ability of antibody to dispose of microorganisms. >In addition to the complement components there are inhibitors of complement which play an important role in regulating the system and preventing complement from damaging our own cells and tissues. |
|
|
|
What is the role of C3?
|
>The 3rd component of complement, C3 is a major serum protein 1mg/ml
>Acts at the heart of the complement system ACTIVATION: >Enzymatic splitting leads to its activation (i.e. it is a zymogen). >Active forms are C3a and C3b >If activation occurs on the surface of a bacterial cell, most of the C3 is in C3b form - Marks (opsonises) bacterium out for phagocytosis because phagocytes have C3b receptors. - Also acts as a focus for other complement components (C5b6789) to collect and form into small pores that cause lysis. |
|
|
|
Draw and annotate the complement pathway.
|
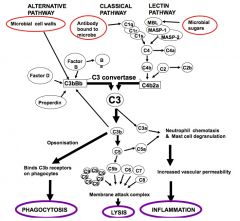
|
ALTERNATIVE PATHWAY:
>Ticks over slowly and is accelerated when it occurs on the microbial surface CLASSICAL PATHWAY: >Involving antibody bound to antigen LECTIN PATHWAY: >Involving the sugars such as mannose on the surface of microorganisms, binding to lectin. EFFECTS: 1. Phagocytosis 2. Cell Lysis 3. Inflammation NB. MASP (MBL associated serene protease), a and b designate the cleavage fragments of complement proteins. |
|
|
Describe the functions of complement.
|
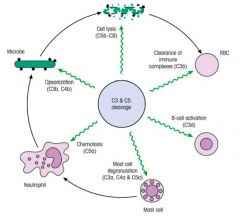
>Opsonisation with C3b is a key event in marking many bacterial and fungal cells for subsequent destruction.
>Lysis by the membrane attach complex is perhaps less crucial for protection against most organisms; important in protecting against Neisseria species. >Other main cleavage fragment C3a has a different function: - with C5a (derived from C5) it promotes inflammation by activating mast cells; - results in vascular permeability increases, drawing neutrophils, monocytes, antibodies and more complement into the tissues; - C5a [and C3a] also function as chemotactic factor for neutrophils attracting them to infection |
NB. RBCs possess complement receptors - picking up bacteria cell materials and transporting them to the liver and spleen for clearance.
|
|
|
What are the major functions of the complement system?
|
1. INITIATION:
>C5a [and C3a] of acute inflammation by direct activation of mast cells. 2. ATTRACTION: >by C5a of neutrophils (chemotaxis) to the site of microbial attack. 3. ENHANCEMENT: >by C3b of attachment of the microbe to the phagocyte (opsonisation). 4. KILLING: >by C9 of the microbe activating the membrane attack complex (C5b6789). |
|
|
|
Detail the three pathways of complement activation.
|
CLASSICAL PATHWAY:
>Requires presence of antibody >Starts with the binding of C1q, C1r and C1s to antibody molecules that have become attached to the pathogen >Activates C4 and C2 to form C4b2a/C4b2b which cleaves C3 --> C3a and C3b >Wherever antibody molecules are bound to antigen there will be C3b - Since phagocytes recognise both C3b and antibody molecules (via receptors) the chances that the bacterium will end up inside the phagocyte are tremendously increased. ALTERNATIVE PATHWAY (second pathway to be discovered): >Occurs spontaneously on the surface of cells such as bacteria >Via factors B, D and properdin (ingenious inhibitors stop it happening on the body's own cells) >C3bBb (key alternative enzyme) converts C3 into C3a and C3b LECTIN PATHWAY: >Similar to the classical pathway but antibody independent >Triggered by microbial surface sugar recognition by Mannose binding lectin (MBL) and subsequent binding to MBL associated serene proteases (MASP-1 and MASP-2). |
|
|
|
How does Epstein Barr virus infect B lymphocytes?
|
>By binding to the complement receptor.
|
|
|
|
Which cells are phagocytes?
|
>Neutrophils
>Monocytes/Macrophages >Dendritic cells >Eosinophils (slightly) |
|
|
|
Describe the process of opsonisation.
|
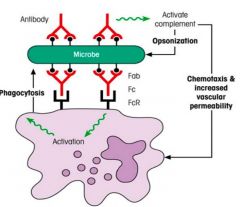
>Opsonisation is the process of making microbe easier to phagocytose.
>Opsonins: - C3b - C4b - Antibody (bridge between innate and adaptive responses) >Mononuclear phagocytes use their surface receptors which bind to C3b or to the Fc region of IgG antibody, to attach to C3b or IgG coating the microbes respectively. |
|
|
|
Describe opsonin binding affinities from weakest to strongest.
|
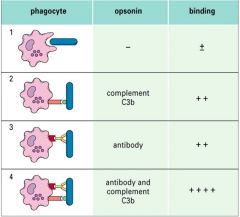
|
|
|
|
Describe the process of phagocytosis.
|
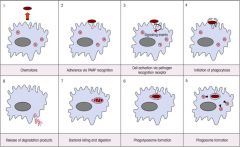
|
>Similar in macrophages and neutrophils.
1. Phagocytes follow chemotactic gradients of microbial or immunological molecules (complement important here) 2-3. Microbe must attach to phagocytic cell via PAMPS on the microbe recognised by PRR on the surface of the phagocyte. - Binding enhanced if complement and or Ab has coated the microbe and is recognised by corresponding complement Ab (Fc) receptors on phagocyte (opsonisation). 4. Membrane movements involving cytoskeleton occur when phagocyte senses microbe 5. Membrane invaginates around microbe into an intracellular vesicle called a phagosome. 6. Phagosome fuses with lysosomes (phagolysosome) containing: 7. O2 radicals, granules possessing various toxic proteins able to kill majority of bacteria/fungi etc; also digestive enzymes, which break down microbe. 8. Degradation particles are released from cell. |
|
|
What is meant by respiratory burst in the context of phagocytosis?
|
>Occurs when phagocyte engulfs a microbe
>Generation of microbicidal oxygen free radicals including: - Superoxide - Hydrogen peroxide - Hypochlorite ions |
|
|
|
What are the three main ways of intracellular killing in phagocytosis?
|
1. Oxidative:
- O2- (superoxide) - H2O2 (hydrogen peroxide) - OH- (hydroxyl radicals) - OCl- (hypochlorite) 2. Nitric oxide related killing: - Reactive nitrogen intermediates 3. Non oxidative killing - Lysozyme - Cationic Defensins - Lactoferrin (absorbs Fe essential for bacterial) - Proteases |
|
|
|
Describe initiation of the acute inflammatory reaction.
|
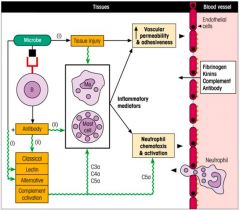
>Connective tissue, skin, lungs and intestine contain mast cells
>When they degranulate they help initiate the acute inflammatory response >Granules contain large amounts of histamine and other molecules that act on blood vessels and smooth muscle. - blood flow increases - damage limitation and control of infection begins >MCs stimulated by C5a and C3a and by antigen (after sensitisation by IgE) >Mast cells :. bound up in innate and adaptive immune responses. NB. Basophils are similar to mast cells. |
|
|
|
Describe the vascular changes in acute inflammation.
|
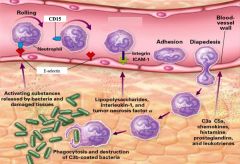
>Inflammatory mediators activate endothelial cells to express adhesion molecules
>PMNs begin rolling as they form weak attachments through their sialyl-Lewis X molecules (S-Lx) to E-selectin on the endothelial surface. >Binding to chemokines released into the blood vessel and displayed on proteoglycan molecules further slows down the PMN down and activates it. >Activation results in changing LFA-1 from its inactive to its active form, which can then bind to ICAM-1 on endothelial surface leading to arrest and flattening of the PMN. >Endothelial cells separate and allow the PMN to squeeze through tight junctions between the endothelial cells (extravasation and into the tissues where inflammatory mediators are being released. NB. Possibly aided by nuclear morphology... |
|
|
Identify this cell type and its function.
|
DENDRITIC CELLS:
>Possess long dendritic processes (similar to nerve dendrites) >Either 'Interdigitating' or 'Follicular' (not formed via haematopoiesis) have little in common aside from morphology >Assist in the recognition of antigen by lymphocytes INTERDIGITATING DENDRITIC CELLS: >'Langerhans cells' in the skin >Found in tissues >Migrate via afferent lymph to T-cell areas (paracortex) of lymphoid organs. >Possess pathogen recognition receptors and are: - Phagocytic initially - Differentiate into antigen presenting mode (expressing large amounts of MHC II - use to process processed antigen to TCRs on Th cells) >Express CD80/CD86+ >Can activate naive T cells (only cell capable of doing this) FOLLICULAR DENDRITIC CELLS: >Found in lymphoid follicles >Non phagocytic >MHC II negative >Store non processed antigen for long periods in the form of Ab-Ag complexes >Display Ag to BCRs on B lymphocytes (not obligatory) >FDCs hold the complexes on their surface using Fc receptors and complement receptors - :. express Fcγr and complement receptors |
|
|
|
Describe the polarisation of T-helper responses.
|
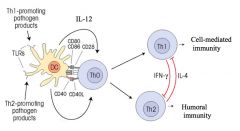
Th1 cell mediated immunity:
>IL-4 downregulates Th1 Th2 humoral immunity: >IFN-γ downregulates Th2 (Depends on PRR) |
|
|
|
Compare the differences between IDCs and FDCs.
|
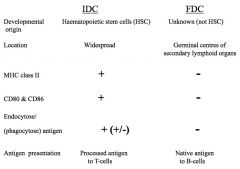
|
|
|
|
Describe the migration and maturation of IDCs.
|
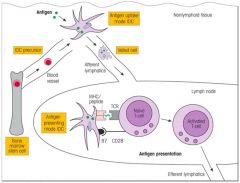
NB. Co-stimulation is necessary for activation of the co-receptor; Activated T cells move to site of infection.
|
|
|
|
How do dendritic cells recognise microbes?
|
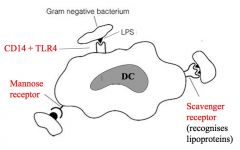
>Via broadly specific PRRs to recognise PAMPs
|
|
|
|
What are toll-like receptors?
|
>A family of 12+ transmembrane cell surface or intracellular proteins
>Are PRRs - recognise various microbial components: - TLR2: Gram +ve peptidoglycan - TLR3: Virus derived dsRNA - TLR4: Gram -ve LPS >Expressed on dendritic cells, macrophages/monocytes |
|
|
|
Identify this cell type and its function.
|
>Lymphocyte (B or T - immunofluorescence is required to identify which)
>Exhibits antigen specificity and immunological memory >On activation in lymphoid tissues, resting lymphocytes develop into larger lymphoblasts. - B lymphocytes may develop into Ab secreting plasma cells LYMPHOCYTES: >Recirculate around the body through various lymphoid organs to encounter specific Ag >Have individual Ag specific receptors in plasmamembrane >Undergo clonal proliferation when activated by Ag making contact with their Ag receptor - allows build up of sufficient numbers of Ag specific cells to deal with infection >Show property of immunological memory giving rise to faster and bigger response on re-encounter with same Ag. |
|
|
|
Describe key receptor/co-receptor differences between B and T cells.
|
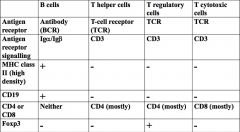
NB. B cells secrete Ab, Th amplify immune responses, Tregs limit immune responses and Tc kill infected cells.
|
|
|
|
What is the difference between soluble Ab and B cell receptors?
|
>Ab lacks transmembrane sequence (hydrophobic sequence of amino acids) and is therefore soluble.
>Otherwise structurally the same (i.e. Ag specific) |
|
|
|
Describe the process of lymphocyte proliferation.
|
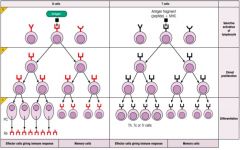
CLONAL EXPANSION OF T-CELLS:
>Selected by specific antigen to take part in immune response (clonal selection) >Proliferate producing a large number of cells (clonal expansion) >First memory cells Tcm (central memory - possess homing and chemokine receptors - recirculate to T cell areas of lymphoid tissues - act as clonal reservoir proliferating on subsequent Ag activation - give rise to Tem (effector memory) which give rise to effector function i.e. cytotoxic activity - migrate to inflamm. sites (+ non lymphoid) - On activation produce IL-2 and receptors (autocrine), other surface molecules include CD40L which interacts with CD40 on dendritic cells producing IL-1 and IL12 (Th inducers) CLONAL EXPANSION OF B CELLS: >Activated by Ag and helped by T cells >Proliferate and mature into memory cells or plasma cells >Memory cells: - Produce Ag for expression on their cell surface and able to respond to reintroduced Ag >Plasma cells: - no cell surface Ab receptors - Ab factories of the same specificity as the Ag receptor on the stimulated parent B cell - Plasma cells produce Ab of one specificity, class and subclass |
|
|
|
What are lymphocyte markers?
|
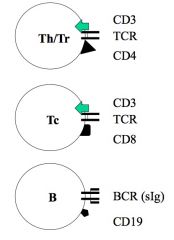
>Cluster differentiation molecules
- Determined by cross inhibition studies - Antigens that differentiate cells NB. all T-cells express CD3; when TCRs recognise pathogen, CD3 signals for activation |
|
|
|
Describe the process of lymphocyte proliferation.
|
CLONAL EXPANSION OF T-CELLS:
>Selected by specific antigen to take part in immune response (clonal selection) >Proliferate producing a large number of cells (clonal expansion) >First memory cells Tcm (central memory - possess homing and chemokine receptors - recirculate to T cell areas of lymphoid tissues - act as clonal reservoir proliferating on subsequent Ag activation - give rise to Tem (effector memory) which give rise to effector function i.e. cytotoxic activity - migrate to inflamm. sites (+ non lymphoid) - On activation produce IL-2 and receptors (autocrine), other surface molecules include CD40L which interacts with CD40 on dendritic cells producing IL-1 and IL12 (Th inducers) CLONAL EXPANSION OF B CELLS: >Activated by Ag and helped by T cells >Proliferate and mature into memory cells or plasma cells >Memory cells: - Produce Ag for expression on their cell surface and able to respond to reintroduced Ag >Plasma cells: - no cell surface Ab receptors - Ab factories of the same specificity as the Ag receptor on the stimulated parent B cell - Plasma cells produce Ab of one specificity, class and subclass |
|
|
|
What is the difference between soluble Ab and B cell receptors?
|
>Ab lacks transmembrane sequence (hydrophobic sequence of amino acids) and is therefore soluble.
>Otherwise structurally the same (i.e. Ag specific) |
|
|
|
Describe key receptor/co-receptor differences between B and T cells.
|
NB. B cells secrete Ab, Th amplify immune responses, Tregs limit immune responses and Tc kill infected cells.
|
|
|
|
Identify this cell type and its function.
|
>Lymphocyte (B or T - immunofluorescence is required to identify which)
>Exhibits antigen specificity and immunological memory >On activation in lymphoid tissues, resting lymphocytes develop into larger lymphoblasts. - B lymphocytes may develop into Ab secreting plasma cells LYMPHOCYTES: >Recirculate around the body through various lymphoid organs to encounter specific Ag >Have individual Ag specific receptors in plasmamembrane >Undergo clonal proliferation when activated by Ag making contact with their Ag receptor - allows build up of sufficient numbers of Ag specific cells to deal with infection >Show property of immunological memory giving rise to faster and bigger response on re-encounter with same Ag. |
|
|
|
What are toll-like receptors?
|
>A family of 12+ transmembrane cell surface or intracellular proteins
>Are PRRs - recognise various microbial components: - TLR2: Gram +ve peptidoglycan - TLR3: Virus derived dsRNA - TLR4: Gram -ve LPS >Expressed on dendritic cells, macrophages/monocytes |
|
|
|
How do dendritic cells recognise microbes?
|
>Via broadly specific PRRs to recognise PAMPs
|
|
|
|
Describe the migration and maturation of IDCs.
|
NB. Co-stimulation is necessary for activation of the co-receptor; Activated T cells move to site of infection.
|
|
|
|
Compare the differences between IDCs and FDCs.
|
|
|
|
|
Describe the organisation and circulation of lymphocytes in the blood.
|
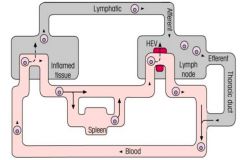
PRIMARY LYMPHOID ORGANS:
>T cells derived from haematopoietic bone marrow stem cells, develop in Thymus >B cells fully develop in bone marrow (Bursa Fabricius) SECONDARY LYMPHOID ORGANS: >T and B cells carry out function here >Include: - MALT (GALT, BALT, NALT) - Lymph nodes - Spleen >Homing receptors on lymphocytes allow them to attach to HEVs so they may enter lymph nodes >T cells circulate once per day >B cells more slowly than T cells >Lymphocytes are only cells in blood stream that can move into and then out of the various body tissues. LYMPHOCYTE RECIRCULATION: >Lymphocytes traffic to secondary lymphoid tissues and continuously from lymph nodes -> efferent lymph -> blood -> (afferent lymph) -> lymph nodes >Enables lymphocytes with specific receptors to come into contact with their antigens localised in the different secondary lymphoid organs and in inflamed tissues. |
|
|
|
What are the antigen recognition molecules for lymphocytes and nucleated cells?
|
Lymphocytes:
>B cell receptor (transmembrane Ab) - Highly specific for native Ag (antigen in its natural conformation) >T cell receptor: - Highly specific for Ag peptide (processed peptide-MHC combination) All nucleated cells: >Major Histocompatibility Complex - Specifically bind a range of peptides (cross reactive) - Some characteristics must be shared e.g. amino acid location or residue charges NB. All three groups of proteins have a common origin in evolution: - members of the Ig superfamily - composed of polypeptide chains with globular domains - tertiary structure results in formation of a structure able to bind a ligand (non reversible, obeying laws of mass-action) |
|
|
|
Describe the morphology and function of the B cell receptor.
|
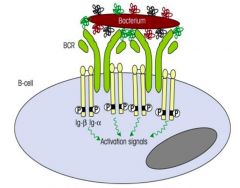
>Consists of transmembrane version of antibody:
- 2 identical H and L chains bound by disulphide bonds - Each polypeptide chain comprises variable and constant region >IGα and IGβ associated with cell surface Ab - These represent ITAMs (immune receptor tyrosine-based activation motifs) - ITAMs become phosphorylated by protein kinases which then activate gene transcription for B cell specificity >Majority of B cells have not encountered their specific Ag: - naive B cells - express monomeric IgM and IgD |
|
|
|
Describe Antibody-Antigen binding.
|
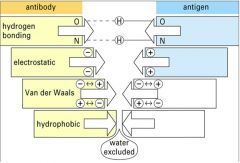
1. Non-covalent:
- h-bonding - van der Waal's bonds - electrostatic forces - hydrophobic interactions 2. Complementary - Ab variable regions bind to complementary Ag epitopes - proteins can be bound by more than one Ab through binding to different epitopes - more complex Ag binds more Ab - hypervariable regions enables recognition of different Ag |
|
|
|
Describe the morphology and function of the T cell receptor (TCR).
|
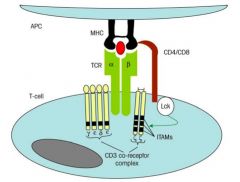
>Most T cells have Ag receptor molecules composed of a TCR αβ polypeptide heterodimer
- Some use γδ as their receptor (epithelial surfaces) - Polypeptide chains have variable V and constant C regions >CD polypeptides (CDγ CDδ CDε together with ζ chains) associated with T cell receptor and are involved in cell signalling (ITAMs) immunoreceptor tyrosine-based activation motifs >T cells express either CD4 or CD8 once mature: - recognises side of MHC >T CELLS RECOGNISE Ag THAT IS: - PROCESSED INTO PEPTIDES - PRESENTED BY MHC |
|
|
|
Describe the pathways for processing antigen for presentation to T-cells.
|
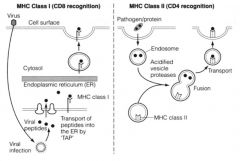
ENDOGENOUS PATHWAY:
>taken from inside the cell e.g. viral proteins >for CD8 recognition >processes peptides into 8-10 aa fragments >MHC I presentation (occurs on all nucleated cells in the body): - Infecting particles are taken up by transporter assoc. with Ag processing (TAP) - Processed at ER - Exocytosed to cell surface for presentation EXOGENOUS PATHWAY: >MHC II - Professional APCs e.g. IDCs, Macrophages, B cells, thymic epithelium - Recognised by CD4 cells >Pathogen endocytosed (e.g. endogenous) - endosome then infused with enzymes - fused with MHC molecule and transported to surface to 'present' >Fragments 15 aas in length |
|
|
|
Describe the structures of HLA 1 and 2 (MHC I and II).
|
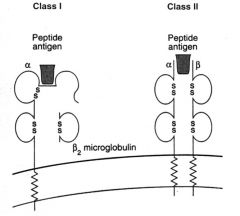
MHC I:
>Consists of two α-helices and a β pleated sheet floor >Two outer domains of the α chain of MHC I form groove for peptide binding - β2 micro globulin essential for correct folding and surface expression of MHC I >Mainly present endogenous Ag e.g. viruses MHC II: >Consists of two α-helices and a β pleated sheet floor >Outer domains of α and β chains form the peptide binding groove >Mainly bind peptides derived from exogenous Ag taken in by phagocytosis |
|
|
|
Describe 3 pathways of Ag processing for T cell presentation.
|
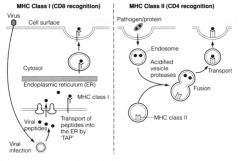
1. ENDOGENOUS PATHWAY
>Viruses can infect any nucleated cell of the body - Viral proteins synthesised by host vital for replication and survival - Viral proteins become degraded in the cytoplasm inside a structural complex of proteolytic enzymes called proteosome - Generates aa 8-10 residues in length - aa transported to ER by TAP - Newly assembled MHC I molecules bind peptides and MHC-peptide complex taken from ER to cell surface - CD8 Tc / CTLs with TCR specific for MHC class I combination can now kill the infected cell 2. EXOGENOUS PATHWAY >Microbes and fragments of are taken into APCs by phagocytosis (MØ, IDCs) or endocytosis (DCs, B cells) - Protein components degraded by proteolytic enzymes (e.g. cathepsins) - On fusion with vesicles containing MHC II molecules, peptides around 15 aa long bind together - MHC II-peptide transported to cell surface - CD4 Th with a TCR specific for the peptide-MHC II combination now activated to help other cells in immune system (e.g. by secreting cytokines) 3. CROSS PRESENTATION >2 pathways above not entirely compartmentalised >Peoptides derived from exogenous Ag can leave the phagocytic vesicle and make their way into MHC-I (endo) pathway to enable DCs to activate CD8+ Tc/CTLs >Reverse also true |
|
|
|
Describe the basic structure of Antibody.
|
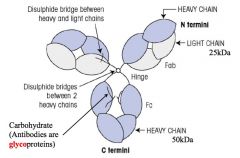
>Composed of heavy and light polypeptide chains held together by interchain disulphide bonds.
>Structure of the transmembrane version of Ab acting as the B cell receptor and the soluble version secreted by the plasma cell that develops from that B cell are identical except that: - soluble version lacks the transmembrane and cytoplasmic sequences - IgM and IgA the monomeric Ig assembles into pentamers or dimers respectively and contain additional proteins associated with their polymerisation and transport (e.g. joining and secretory proteins) |
|
|
|
What are antibodies and where are they found?
|
ANTIBODIES = Immunoglobulin and are glycoproteins
>FOUND in CELL SURFACE (transmembrane) and SECRETED (soluble) versions. >ONLY MADE by B CELLS - Synthesise a transmembrane version which functions as BCR for antigen >AND BY PLASMA CELLS: - these are derived by these B-cells following activation by antigen - plasma cell produces a soluble version of antibody of identical antigen specificity to that of the original B cell from which they were derived NB. Ab can be found on the surface of many other cells of the body either because: - Fc receptors have bound the constant region of the heavy chain of the antibody - because the Ab V region has specificity for antigen (e.g. viral antigen on the surface of a cell) |
|
|
|
Draw a schematic of an antibody, describing the functional parts of the molecule.
|
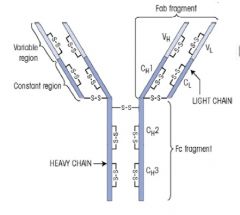
There are two functional parts to the Ab molecule.
1. FRAGMENT ANTIGEN BINDING: >Fab comprises the arms of the antibody and includes the variable region which differs from one Ab to another. >The variable region gives the Ab its specificity and recognises a particular antigenic shape. - Epitope = precise part of antigen recognised by the antibody >Fab region contains Variable domain and the first Constant domain of one of the heavy chains and all VL and CL of one the light chains - Each Ab has two arms :. two Fab fragments FRAGMENT CRYSTALLISABLE: >Fc contains biological activity effector part of antibody >Composed of the remaining sections of the 2 heavy chains (CH2 and CH3 for IgG, A and D, and CH2-4 for M and E. >Where molecule fixes (binds and activates) complement, and attaches to Fc receptors on cells. >Constant region of th heavy chain determine the Ab class and is structurally adapted for a particular biological activity in each class >Different classes of Ab function best at different sites of the body >2 Fab arms in Ab are identical - True for 4 Fab in dimeric IgA and 10 Fab in pentameric IgM. |
|
|
|
What is the difference between intrinsic and functional affinity of Ab?
|
INTRINSIC AFFINITY:
>Strength with which an antigen-binding arm binds an epitope. FUNCTIONAL AFFINITY/AVIDITY: >Ab attaches to >1 identical epitopes on antigen binding strength is increased. |
|
|
|
How many different classes of Ab are they? List them, their Heavy Chain and Light Chain and their location in the body.
|

>5 classes of Ab
>Based on Isotypes of the heavy chains used >2 light chain isotypes >Only one light chain and one heavy chain isotype NB. both H+L chains are identical in Ab molecule. |
|
|
|
Describe the structure and role of IgM.
|
>Two configurations:
- monomeric form on the surface of naive B lymphocytes - pentameric in circulation as soluble 5 Ab monomers joined by J chain >970kDa :. largely confined to circulation >Predominant in early immune responses to most Ag >First line defence against bacteraemia (bacteria in blood) >Although IgM Fabs have low affinity, pentameric structure gives it relatively high avidity >Multiple binding sites of IgM allow it to attach simultaneously to several identical epitopes on different particulate Ag (e.g. bacteria) leading to their agglutination. >Blood group Ab are mainly this class. |
|
|
|
What is the function of Ab?
|
Neutralising function:
>Bind toxins to prevent binding to cells >May bind virus receptors Enhancement function: >Act to focus other parts of the immune system |
|
|
|
Describe the structure and function of IgG.
|
>Predominant immunoglobulin in the bloodstream and tissue fluids
- combates microorganisms and their toxins >4 subclasses (all monomeric) - IgG Ab generally have a higher affinity for Ag than IgM - Relatively small (150kDa) - Readily able to pass from blood to tissues >IgG only class to cross placenta - active process, mediated by a placenta-associated receptor called FcRn - provides neonate with important protection against infections to which the mother is immune |
|
|
|
Describe the structure and function of IgA.
|
>2 subclasses (1+2) and two forms:
- Monomeric - Dimeric / secretory IgA, linked by a J chain >Dimeric IgA: - Transported across epithelial surfaces and released - with a protein called secretory component attached to it into the exterior lumen - Secretory IgA provides the primary defence against local infections - Found in saliva, tears, bronchial secretions, nasal mucosa, prostatic fluid, vaginal secretions and mucous secretions of the small intestine - IgA Ab in breast milk colostrum protect neonates during early life |
|
|
|
Describe the structure and function of IgE.
|
>IgE is a monomer
>Present usually in very low levels in circulation >Mainly found bound to high affinity IgE receptor (FcERI) present on mast cells underlying mucosal surfaces. >IgE mediated degranulation of mast cells on exposure to its Ag enhances acute inflammatory response by recruiting a variety of cells into the site of infection. >Circulating levels of this Ab are increased during parasitic worm infections - potentially evolved against these intruders >Negatively, IgE responsible for symptoms of atopic allergy, resulting in enhanced acute inflammation. |
|
|
|
Describe the structure and function of IgD.
|
>Present as monomer on surface of naive B cells (along with M)
>Acts as Ag receptor for control of lymphocyte activation - Like IgE it is present only at very low levels in the circulation. |
|
|
|
In order of concentration, list the different classes of Ab, and their function (include subclasses if possible).
|
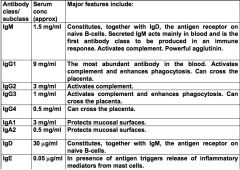
NB. Ab are not able to penetrate living cells - only protective against extracellular microbes (mainly bacteria)
>Short half life in the serum: - 21 days for IgG - 10 days for IgM - 6 days for IgA |
|
|
|
Describe the effect of multivalency on Ab binding to Ag.
|
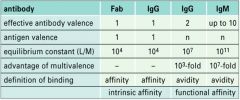
|
|
|
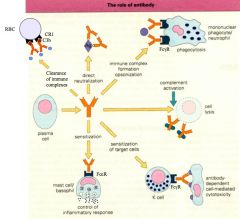
Which Ab is responsible for which process?
|
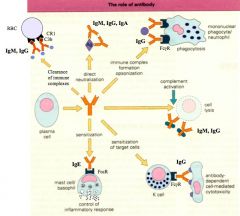
|
|
|
|
What is an immune complex?
|
>Combination of antigen and antibody
- When Ab binds Ag they often 'fix' complement via the Classical Pathway, so that immune complexes also contain complement. |
|
|
|
Is the innate response always enough to dispose of a pathogen?
|
>Not always, hence the adaptive response.
>Adaptive system will come into contact with pathogen leading to adaptive immunity. |
|
|
|
Describe the primary and secondary immune response to Ag.
|
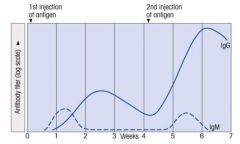
>IgM is relatively comparable for each response.
>IgG proliferate much more upon the secondary infection. |
|
|
|
Explain the process of clonal selection.
|
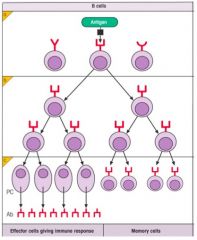
>Following Ag entry into body:
- becomes localised in secondary lymphoid tissues (e.g. lymph nodes and spleen) - attaches to Ag receptors on B cells - T independent Ag (often polysaccharides) directly activates the B cell to make Ab only of IgM class - Protein Ag do not usually activate specific B cells unless Th cells are present to 'help' (:. T-dependent) >Th cell activation - requires protein antigen processing by APCs e.g. dendritic cells or macrophages - subsequently requires Ag presentation to B cells - Ag then selects specific B cells to take part in the Ab response >Clonal selection: - Selected B cells undergo rapid clonal proliferation in order to expand up the number of Ag specific cells to a sufficient level to deal with the infecting pathogen. >Some of the cells of the expanding clone become memory cells - many differentiate into plasma cells which secrete large amounts of specific Ab - Th cell interaction with the B cell also induces class switching |
|
|
|
Draw and explain the shape of the primary Ab response.
|
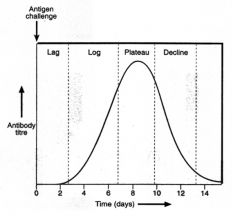
>Ag processing, presentation and clonal selection and Ab production take time
- results in a lag period before significant levels of Ab are detectable - after a couple of days increasing amounts of Ab are made as more and more B cells divide and give rise to plasma cells - Leads to log phase as Ab increases in circulation - Subsequent plateau in which Ab has bound free Ab so that lymphocytes are no longer stimulated >Ag removed and Ab response subsides, declining Ab in serum. NB. Most plasma cells die after carrying out their function. |
|
|
|
What is meant by class switching w.r.t. Ab?
|
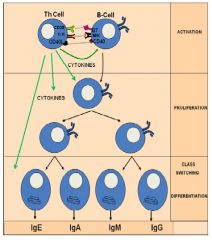
>Naive B cells coexpress IgM and IgD
>Following activation by Ag, most B cells class switch to produce IgG, A or E - Requires help from Th: 1. cell cell interactions e.g. CD40-CD40L 2. Cytokines e.g IL-4 and IL13 cause class switch to IgE >First Ab to be produced in the primary response are IgM: - IgM B cells are stimulated to divide and give rise to plasma cells secreting IgM - Additionally, under the influence of cytokines secreted by Th cells, some of the B cells switch to IgG, A or even IgE production during their proliferation, some developing into memory cells. >Since microbes have many different Ag each with several epitopes (the actual structures on the Ag recognised by Ab) the Ab response is said to be polyclonal. |
|
|
|
Which interleukins cause class switching to IgE?
|
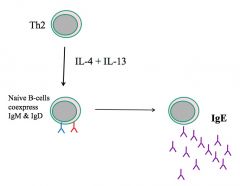
|
|
|
|
What is the structure of an Ab light chain?
|
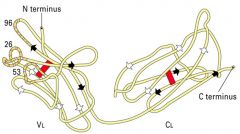
>Contains hypervariable regions
- Complementarity determining regions exist on the Variable regions - Bind to Ag (hence complementary) |
|
|
|
What are the subclasses of Ig?
|
>IgG = 1-4
>IgA = 1+2 |
|
|
|
What are Ag allotypes?
|
>Minor inherited genetic polymorphisms on immunoglobulins e.g. Km (on K light chains) and Gm (on IgG heavy chains)
|
|
|
|
What are the different classes of Ab and their functional structures.
|
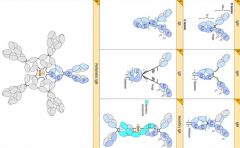
>IgM 5 units joined by J chain (found in circulation and difficult to move elsewhere due to size of 970kDa)
>IgA = dimer, found in mucosal surfaces |
|
|
|
Explain the Ab-Ag reaction.
|
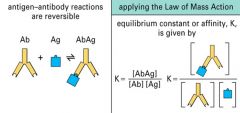
>This is a reversible reaction
>Equilibrium is formed and binding affinity may be calculated |
|
|
|
Which cellular events take place in the germinal centre of secondary lymphoid tissues?
|
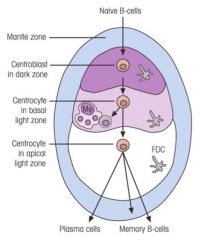
1. Proliferation
2. Class switching 3. Affinity maturation |
|
|
|
How do T cells support Ab production?
|
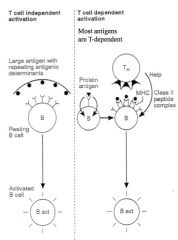
>Critical role in B cell activation for majority of Ag (T-dependent)
>Cell-cell interactions required for initial activation of B cellse.g. - CD40L on T cell and CD40 on B cells - CD28 and B7 molecules on B-cell >Cytokines also involved in driving B cell proliferation, class switching and differentiation into plasma cells - IL4 causes B cells to switch to IgE production |
|
|
|
Which cellular events take place in the germinal centre of secondary lymphoid tissues?
|
1. Proliferation
2. Class switching 3. Affinity maturation |
|
|
|
Which cellular events take place in the germinal centre of secondary lymphoid tissues?
|
1. Proliferation
2. Class switching 3. Affinity maturation |
|
|
|
What is meant by immunological memory?
|
>Adaptive immune response 'remembers' that it has encountered Ag in the past
>Memory cells (specific cells of B cell clone) are present post primary response - more available for subsequent responses to the same Ag >Secondary response is faster at bigger - in addition to quant. changes in men response and class switching also 'affinity maturation of Ab' NB. Little or no memory response in the absence of Th cells |
|
|
|
What occurs after B cells become activated?
|
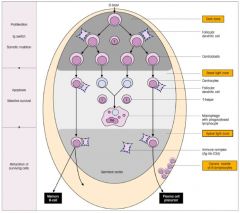
>B cell activation leads to germinal centres which develop in secondary lymphoid tissues.
>These are sites where B cells undergo: - clonal expansion - class switching - somatic hypermutation of Ig variable region genes - selection of high affinity clones leading to affinity maturation of Ab response. >Following antigenic stimulation of B cell and during switch from IgM to IgG (A or E) production, there is a high rate of hypermutation in genes coding for variable domains (esp. in CDRs) - since these are random, occasional cells are produced with receptors able to bind more strongly to Ag - These are rescued through recognition of the Ag present in form of immune complexes bound to FDC surfaces; continue to proliferate with some becoming memory cells and others developing into plasma cell precursors - Plasma cell precursors leave the germinal centres and become plasma cells in the medulla of the lymph nodes or splenic red pulp - conversely receptors will be produced that bindles well to Ag, and insufficient to stimulate B cells; - these B cells are of no use to the secondary response and apoptose and are removed by macrophages |
|
|
|
Explain the importance and characteristics of the mucosal immune system.
|
>Most infections initially take place at mucosal surfaces.
- Most immune responses occur outside of the blood - Challenging to study mucosal immunity >Characteristics of mucosal defence: - Mucus* - Gastric acidity* - Proteolytic enzymes* - Tight junctions between epithelial cells* - Defensins produced by Paneth cells (neutrophils) at base of the crypts - Neutrophils recruited if PAMPs detected *Important in protecting but not technically part of immune response |
|
|
|
Explain the acquisition process of IgG and IgA from maternal breast milk by neonates.
|
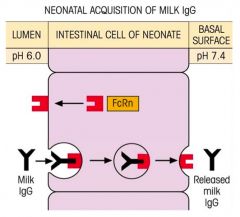
>GAGA
>Maternal IgG is important in protecting the foetus - Breaks down shortly postpartum (4-6 weeks) >Process: - IgA remains on luminal surface - IgG binds to FcRn receptor on luminal membrane; triggering endocytosis of IgG (pH dependent 6.0 at lumen) - Upon reaching basal surface, exocytosis occurs and pH of 6.4 triggers dissociation of IgG from FcRn - FcRn recycled |
|
|
|
Describe the overall structure of the mucosal immune system.
|
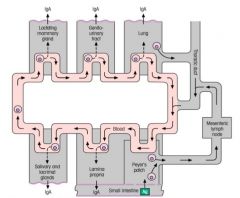
Mucosal Associated Lymphoid Tissue:
>50% of all lymphoid tissue >Comprises, BALT, NALT and GALT - Subepithelial accumulations of lymphoid cells unconstrained by connective tissue capsule - May occur as diffuse collections of lymphocytes, plasma cells, dendritic cells and macrophages throughout lung and lamina propria of intestinal wall - MALT forms a separate interconnected secretory system within which cells committed to IgA or IgE synthesis may circulate >Acts in relative isolation 'self contained' >Cells move from one surface to another however |
|
|
|
Describe the structure of secretory IgA.
|
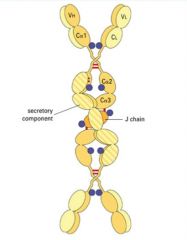
>Dimeric secretory IgA present at mucosal surfaces
- produced by plasma cells in lamina propria - Ab transported across epithelial cells into lumen of tract via poly Ig receptor >Monomers bound by J (joining chain) |
|
|
|
What are the functions of secretory IgA?
|
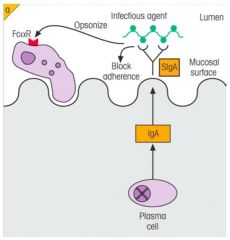
>Prevents pathogen adherence
>Opsonises bacteria - Macrophages and neutrophils possess FcαR which bind IgA |
|
|
|
Explain IgE's role in mucosal immunity.
|
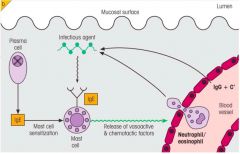
>Lamina propria is rich in mast cells
- thought to be important to protect against parasitic worm infections - since main Ab against worms are IgE, cross linking of IgE on surface of mucosal mast cells by worm Ag leads to mast cell degranulation producing a powerful acute inflammatory reaction >IgE binds to mast cells via FcEr causing release of inflammatory mediators - IgG, cytokines |
|
|
|
What is the role of Intraepithelial lymphocytes?
|
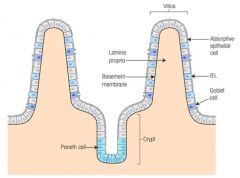
Localised cells on the surface of intestine
>IELs are predominantly T cells - unlike other T cells, IELs do not need priming - upon encountering antigens, they immediately release cytokines and cause killing of infected target cells - express γδ heterodimers >Paneth cells produce cationic defensins |
|
|
|
Explain how gut immune responses are induced.
|
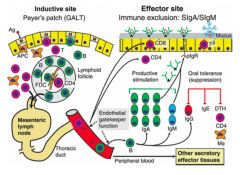
>Induced in Peyer's patches
>Microfold cells interspersed amongst epithelial cells - smaller, shorter distance for Ag to travel - sampling cells >B lymphocytes once activated do not mature into Ab secreting cells in PP but migrate to mesenteric lymph nodes - Undergo differentiation / division - home back to lamina propria - become IgA forming cells protecting intestine with Ab >Therefore PP are inductive sites; lamina propria are effector sites >Induced lymphocytes also appear in lung lymphoid tissue and in other mucosal sites, guided by specific homing receptors with appropriate vascular addressins - e.g. MADCAM-1 NB. Lymphocytes home to different areas in the body - γδ receptor T cells home to mucosal epithelium - B cells move to mucosal tissues secreting IgA G and M - Nasal mucosal cells home to the vagina; interesting for HIV vaccine (vaginal immunity desirable) |
|
|
|
Describe how Ig is transferred to the gut lumen in non neonates.
|
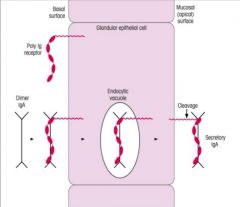
>Via the poly Ig receptor
- only used once (c.f. FcRn) >Receptor destroyed by enzyme - cleavage fragment of receptor is secretory component - Poly Ig receptor not recycled - it is thought that secretory component inhibits intraluminal destruction of IgA via reduction of proteolytic enzyme degradation |
|
|
|
Why doesn't our mucosal immune system respond to food Ag or commensals?
|
>This isn't fully understood
>Allergies can sometimes manifest themselves but they are relatively rare |
|
|
|
Which T cell type activates microbicidal killing by macrophages? How?
|
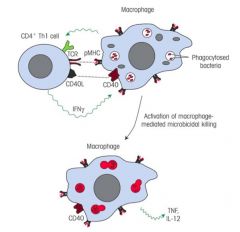
>CD4+ Th1 cells (IFN-γ producing; NOT IL-4)
>Macrophages require co-activation, PAMP stimulation of PRRs aren't sufficient to induce killing >CD4+ T cells recognise small peptides bound to MHC II molecules >Such peptides can be derived from intramacrophage microbes e.g. M. Tuberculosis - On binding to MHC class II peptide complex on the macrophage surface, the T cell is stimulated to release cytokines (IFN-γ) activating the macrophage to be effective in killing the microbe |
|
|
|
What are Th1 cells more likely to support as opposed to Th2 cells in aiding the cell-mediated response?
|
>Th1 cells:
- Support macrophages in removing intracellular microbes >Th2 cells: - Help B cells to make Ab NB. Production of some classes of Ab are helped by Th1 cells |
|
|
|
What is the role of Th17 cells?
|
>Th17 cells produce and secrete IL-17 and IL-22 which promote tissue inflammation
|
|
|
|
What is the role of cytotoxic T cells in cell mediated immunity?
|
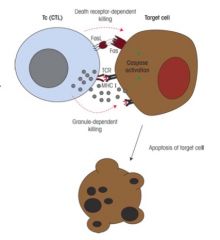
>CTLs mediate apoptosis via two pathways:
- Fas/FasL - Granzyme/perforin pathway >CD8+ T cells recognise small peptides produced inside cells which bind to and are transported tot he surface with MHC I - e.g. virus peoptides >Once Ag receptor on T cell has recognised the MHC peptide complex on virus infected cell; - T cell develops into a fully functional cytotoxic cell which can then kill cells infected with same virus by inducing apoptosis |
|
|
|
Describe the difference between the Fas/FasL and granzyme/perforin pathways.
|
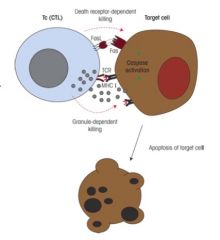
>CTL activity related, occur upon MHC I activation of CTLs
Granzyme/Perforin: >CTL produces perforin similar to MAC of complement; - punches hole in infected cell >Granules containing enzymes called granzymes are produced by CTLs and injected into target cell though perforin channels >Once inside target cell granzymes activate other enzymes called caspases which mediate apoptosis. Fas/FasL: >CTL interaction of FasL (on Tc surface) with Fas on target cell - causes activation of caspases in infected cells by death receptor dependent pathway - leading to apoptosis of infected cell |
|
|
|
How are apoptotic cells removed?
|
>By macrophages, via phagocytosis
>Immunopathology can occur if this reaction is protracted |
|
|
|
How do Th cells support CTL activation?
|
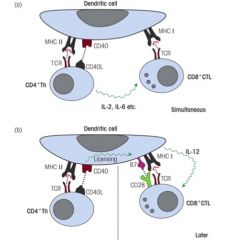
>CD4 Th cells secrete IL-2 and IL-6 which activate CD8 T cells
>CD4 Th cell changes the configuration of dendrite cells to allow CTL activation via ILICE >CD8 T cell binds CD80 and CD86 (B7.1 and B7.2) |
|
|
|
How do T cells mainly regulate other cells?
|
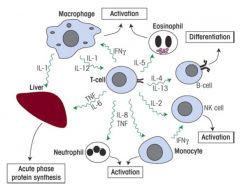
>Via cytokines:
- analogous to hormones but confined to immune system - cytokines are tailored to the required response - may have several different effects on different cells and even at different dose levels on the same cell - different cytokines can have same effect on a particular cell and frequently act tighter to alter each other's activities >Thus most cells respond to a mix of cytokines which may change from moment to moment NB. Cytokines may be used therapeutically |
|
|
|
What cytokines do Th1 and Th2 cells secrete exclusively?
|
Th1: IFN-γ
Th2: IL-4 |
|
|
|
Which cytokines are secreted by different T cell populations?
|
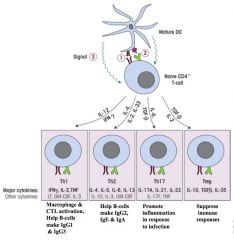
NB. Dendritic cell messengers drive differentiation of naive CD4 cells.
|
|
|
|
What types of molecules are considered cytokines?
|
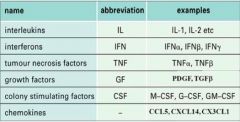
NB. some cytokines e.g. CSFs are not limited to immune system and also play a role in haematopoiesis.
|
|
|
|
Describe different types of network interactions that cytokines can have.
|
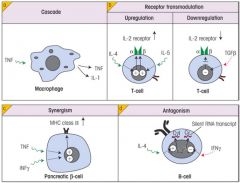
1. Cascade
2. Receptor Transmodulation 3. Synergism (e.g. Type I diabetes) - Excessive cytokines upregulate MHC II causing presentation of self-antigen 4. Antagonism |
|
|
|
Describe some main IL types and their sources and activities.
|
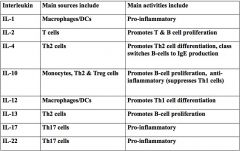
|
|
|
|
What are interleukins?
|
>Cytokines [which have had their nomenclature standardised] which work between white blood cells
>IL-1-27: - Produced by many cell types, but particularly by Th (except IL-1 and IL-12 produced by Macrophages and Dendritic cells) >Each interleukin has multiple activities |
|
|
|
What are CSFs?
|
>Colony stimulating factors
>Mainly responsible for maturation of various kinds of white blood cells in the bone marrow. >Examples include: - Granulocyte-macrophage colony stimulating factor: early myeloid development >Monocyte/Macrophage colony stimulating factor: induces monocyte differentiation >Granulocyte colony simulating factor: induces granulocyte differentiation |
|
|
|
What are TNFs?
|
>Mainly involved in inflammation, showing considerable overlap with IL-1
>Effect: - Macrophage activation - Induction of endothelial cell adhesion molecules (plays a role in acute inflammation) - Stimulates the production of other cytokines NB. Anti-TNF used in rheumatoid arthritis |
|
|
|
What are interferons?
|
>Three main forms:
- IFN-αβ produced by most nucleated cells of body; limit viral replication - IFN-γ produced mainly by Th1 cells and NK cells as well as inhibiting viral replication it can activate macrophages and down regulate Th2 cell activity |
|
|
|
What are chemokines?
|
>Chemotactic cytokines involved in cell recruitment and localisation.
>Fours families group according to arrangement of cysteines in their structure - CXC - C - CX3C - CC |
|
|
|
What are examples of useful and harmful cytokines?
|
Useful cytokines:
>IFN-α: - Treatment of chronic hepatitis C infection, Kaposi's sarcoma and hairy cell leukaemia >IL-2: - Treatment of renal cell carcinoma and metastatic melanoma >CSFs: - Treatment of bone marrow failure; promote development of new phagocytes following haematopoietic stem cell transplantation. Harmful cytokines: >TNF: - Excessive production can lead to a severe drop in blood pressure and death >IL-4 and IL-5: - Allergy |
|
|
|
What are transforming growth factors?
|
>TGF-β has an inhibitory effect:
- Inhibits macrophage and dendritic cell activation - Inhibits growth of B and T cells |
|
|
|
What is the difference between regeneration and repair?
|
REGENERATION...
>...occurs in tissue with labile and stable cells >Only if the supporting architectural framework (i.e. basement membrane) intact - Therefore pure regeneration relatively rare >Initiates at edge of damage from viable tissue - progresses centrally over time ...whereas REPAIR... >...is the replacement of injured cells by fibrous connective tissue >Damaged area of tissue becomes a 'scar' >Occurs when supporting framework of labile or stable tissues destroyed (e.g. basement membrane) >Or when permanent cells have been destroyed >Peripheral, progressing centrally over time |
|
|
|
What different types of healing are there?
|
>Primary intention
- edges of wound opposed e.g. surgical wound >Secondary intention - edges of wound not opposed e.g. burn >Healing of bone fracture |
|
|
|
What are the different types of fracture?
|
>Complete or incomplete
>Closed simple (intact skin) >Compound (fracture site communicates with skin surface) >Comminuted - bone is splintered >Displaced - ends of bone at fracture sites not aligned >Pathological (e.g. cancer) >Stress - following exposure to repetitive loads |
|
|
|
What factors effect healing?
|
LOCAL FACTORS:
>Infection - Most common cause of delayed healing >Mechanical factors - Early wound movement or constant disruption to healing process >Foreign material: - Cannot be phagocytosed and can cause infection >Size, location and type of wound - Small wounds heal quicker than large - Epithelial tissues quicker than connective tissues - Richly vascular tissues quicker than poorly vascular SYSTEMIC FACTORS: >Nutrition - Protein, Vitamin C and Zinc deficiency >Diabetes Mellitus - Increases risk of infection and leads to poorer overall cell function >Circulation - Atherosclerosis and other cause of vascular stenosis reduce vessel flow >Glucocorticoid (steroid drugs) - Steroid have an anti-inflammatory action and impair collagen synthesis >Immunosuppression - Either reduced or poorly functioning leucocytes >Stress (study conducted in medical students) |
|
|
|
What are examples of healing complications?
|
>Wound ulceration due to poor vascularity (e.g. PVD)
>Wound dehiscence (due to increased pressure on scar) >Stretching of scar, due to increased pressure on scar >Excessive growth: - Keloid scars - Exuberant granulation tissue >Contracture - Exaggeration of normal wound contraction - Often occurs due to circumferential scarring - Affects breathing, blood flow and flexion |
|
|
|
Describe the process of primary intention healing.
|
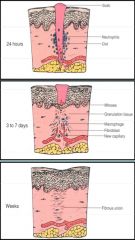
>Immediately - 3 days:
- Haemostasis and fibrin clot formation with scab on top to protect process - blood contacts collagen - platelets activated and fibrin clot forms - cytokines and chemokines attract neutrophils, macrophages which infiltrate the clot >By day 5: - Formation of granulation tissue with fibroblasts and vascular proliferation - Collagen deposited - VEFG released - Regeneration of overlying epithelium, modulated by PDGF and TGFB, contact epithelium and differentiation of fibroblasts to myofibroblasts which enable wound contraction >1 month: - Granulation tissue slowly replaced by fibrous tissue - Collagen fibres realigned along tension lines, replacement of type three collagen by type I |
|
|
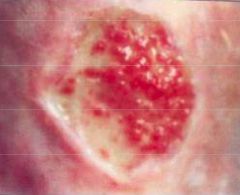
What type of healing will occur here and what is the healing process?
|
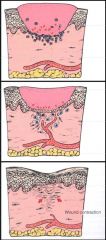
>Similar to primary intention but the edges of wound aren't opposed.
1. Clot forms in a crater 2. Neutrophils and macrophages infiltrate the clot 3. Granulation tissue formed 4. Because of the large defect, the fibroblasts contract and draw the edges of the wound together |
|
|
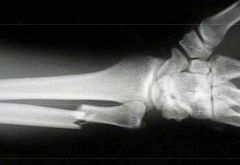
Which type of fracture is this?
|
Displaced
|
|
|
|
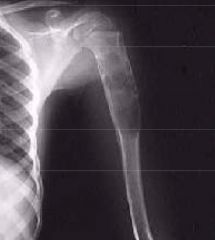
What type of fracture is this?
|
Comminuted
|
|
|
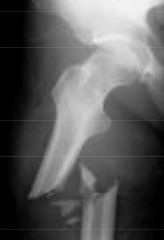
What type of fracture is this?
|
Pathological
|
|
|
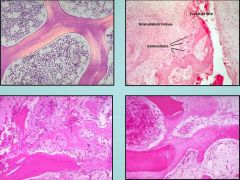
Clockwise from top left identify these four bone morphologies.
|
1. Normal lamellar bone
2. Fracture 3. Remodelling 4. Woven bone |
|
|
|
Describe the process of bone healing in fractures.
|
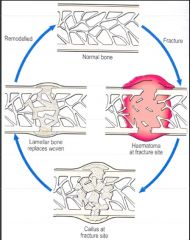
>Bone capable of regenerating completely even if framework is entirely lost.
1. Bleeding from ruptured vessels - clot formation fills wound gap and surrounds area 2. 24-48hours - Neutrophils and macrophages migrate from incision margin, phagocytose debris 3. by day 4: - Formation of granulation tissue - Proliferation of cartilage cells from edge of wound - Cartilage and granulation tissue fill wound site - Known as procallus 4. During 2nd week: - Cartilage begins to calcify - Osteoblasts begin laying down osteoid - First form fibrocartilaginous callus, then a bony callus which is weak as composed of woven bone 5. 2-7 weeks - Remodelling of bony callus - Osteoclastic and osteoblastic activity - Lamellar (organised) bone replaces woven bone - Marrow cavity restored - Bone strength returns to normal - No macro/microscopic evidence of injury |
|
|
|
What is healing and what occurs during healing?
|
>Process in which skin/tissue repairs itself
>Replace of injured cells by parenchymal cells of same type >Healing is preceded by haemostasis >May be achieved via two different processes (often in combination): - Regeneration - Repair STAGES OF HEALING: 1. Haemorrhage, haemostasis 2. Inflammation (cytokines and growth factors) 3. Granulation tissue; - New vessel formation - angiogenesis via VEGF - Fibroblasts - Macrophages (cytokines and phagocytosis) >Scar formation >Remodelling and wound strength |
|
|
|
How is healing controlled?
|
Growth factors:
>Produced by fibroblasts, platelets, macrophages, endothelial cells - epidermal growth factor, cytokines - PDGF (platelet derived growth factor) Cell-cell and matrix interactions: >Contact inhibition - turnover decreases upon contact - density depended regulation of cell growth |
|
|
|
What is meant by tissue regeneration?
|
>Replacement of injured cells by parenchymal cells of same type
>Damaged area of tissue becomes 'normal' 3 CELL TYPES: >Labile: - Cels that constantly divide to replace like cells that are being lost - e.g. skin epithelial cells, gut epithelial cells - bone marrow cells >Stable cells: - Cells that have a low level of replication under normal circumstances - May switch to rapid replication with the correct stimulus - Epithelial cells of kidney, liver, other organs - Fibroblasts, smooth muscle >Permanent cells: - Cells incapable of organised replication - Neurones, Skeletal and Cardiac muscle - Do not regenerate, heart forms scar tissue; brain doesn't undergo scarring, but instead liquefactive necrosis (macrophages phagocytose tissue, leaving a cystic space filled with CSF) |
|
|
|
Why is it necessary to generate diverse antigen receptors?
|
>In order for immune response to be mounted, lymphocytes need to sense the presence of a pathogen
- Pathogen specific - Each lymphocyte specific for one Antigen (occasionally for a small number of different but related antigens) >Pathogen detected via the antibody receptor (BCR) or the T-cell receptor (TCR) |
|
|
|
What is the difference between TCRs and BCRs?
|
>Both:
- Glycoprotein molecules - Encoded by genes >BCRs: - Two identical heavy chains disulphide bonded to each other - Two identical light chains (κ or λ) disulphide bonded to heavy chains >TCRs: - Heterodimers consisting of disulphide linked αβ or γδ polypeptide chains |
|
|
|
How antigen recognition variability brought about?
|
>Each Ab chain possesses variable region (VR) with Ag specificity and constant region (CR)
- CR encoded by constant region gene segment - Variable region coded by 3 (for heavy chains) or 2 (for light chains) gene segments - These segments are referred to as: V (variable), D (diversity), J (joining) |
|
|
|
How does the immune system get around the challenge of multiple pathogens given that there is lymphocyte specificity?
|
>Huge diversity is required
>Not enough DNA in cells for each Ab specificity to be encoded in separate genes >Discovered that BCR and TCR diversity is brought about by a unique process of GENE RECOMBINATION of 3-4 separate gene segments |
|
|
|
Describe the structure of Ig gene loci.
|
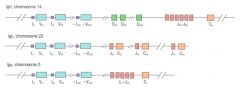
>VDJ segments are located 3x
- 1 set for heavy chains (C14) - 1 set for κ light chains (C2) - 1 set for λ light chains (C22) >Inherited in non recombined 'germ line' form through the germ cells in the normal way |
|
|
|
What is the configuration of V, D and J segments on the chromosome? How does recombination occur?
|
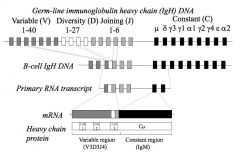
>There is a pool of approximately 40 V segments available for use by the heavy chain and a similar number for light chain
- actual number of segments varies between individuals RECOMBINATION: >In B cells, one IgV genes will be chosen at random to undergo recombination with one out of several J and (for heavy chains) one out of several D gene segments - In all other cells in the body the Ab genes remain in the germ line configuration >Similarly it is only in T cells that TCR genes undergo recombination |
|
|
|
How is recombination in B cells mediated?
|
>Recombinase enzymes mediate the process
>Product of RAG-1 and RAG-2 >RAG enzymes recognise a stretch of nucleotides called a recombination signal sequence (RSS) which flanks the V, D and J gene segments - RSS consist of highly conserved hep tamer, no namer sequences separated by non conserved 12 or 23 base pair spacers >Similar processes apply to TCR gene recombination |
|
|
|
What is a simple way of explaining recombination in B cells?
|
>3 packs of cards
- V, D and J - One card from each pack selected at random |
|
|
|
What are RSSs?
|
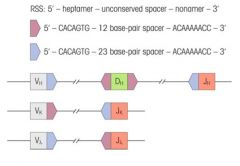
>Recombination signal sequences
>Flank V, D and J gene segments >Consist of highly conserved heptamer - nonamer sequences separated by non conserved 12 or 23 base pair spacers >Similar processes apply to TCR gene recombination |
|
|
|
What are CDRs?
|
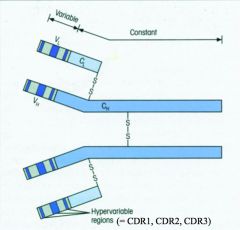
>Within the variable region of the Ab, there are three regions which show exceptional variability
- These are complementarity determining regions - 3 on heavy chain - 3 on light chain >Complementary to and recognise the Ag >Coded by: - V gene segment for CDR1, and CDR2 - VDJ (or VJ for light chain) gene segments for CDR3 :. more diverse |
|
|
|
What is the 12-23 rule and its purpose?
|
>Forms the basis for RSS (recombination signal sequences)
- Only a pair of dissimilar spacer RSSs are efficiently recombined (i.e. one with a spacer of 12 nucleotides will be recombined with one that has a spacer containing 23 nucleotides >Enforces the right type of recombination |
|
|
|
In which 8 ways can Ab and BCRs diversify genetically?
|
1. Multiple germ line genes
2. V-D-J (H) and V-J (L) recombination 3. Recombinatorial inaccuracies 4. N-nucleotide addition 5. Different H+L combinations 6. Class switching 7. Somatic hypermutation 8. Transmembrane or secreted configuration |
|
|
|
What is AID and what is its role?
|
>Activation Induced Cytidine Deaminase
>Patients with an immune deficiency in which AID is mutated (one cause of Hyper IgM syndrome) don't have: - Class switch recombination (CSR) - Somatic hypermutation (SHM) |
|
|
|
What is typically expressed on naive B cells? Why?
|
>IgM and IgD
- The constant region genes define the istotype of the Ab >Before Ag is encountered, they are referred to as naive B cells - These cells co-express IgM and IgD with the same variable region - The co-expression of 2 different classes of Ab is brought about by the production of long primary RNA transcript which includes the VDJ region and the constant region for both IgM and IgD. |
|
|
|
What is meant by recombinatorial inaccuracies?
|
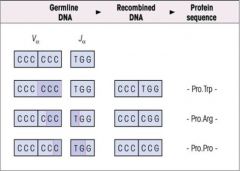
>e.g. Errors in transcription of recombined DNA...
|
|
|
|
What is meant by N-nucleotide addition?
|
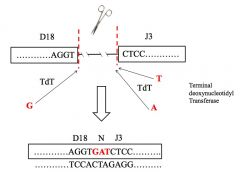
>Addition of another nucleotide(s) by Terminal deoxynuclotidyl transferase (TdT)
|
|
|
|
Explain the process of Class Switch Recombination.
|
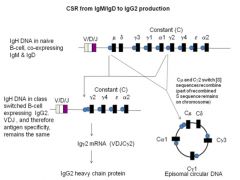
>B cells can switch from initial production of IgM and IgD to production of G, A or E.
>Second recombination process - Analogous to VDJ recombination - Already recombined VDJ sequence is maintained but placed next to either a Cγ, Cα or Cε constant region gene - :. specificity of Ab stays the same, but class changes (e.g. from IgM streptococcal Ab to an IgG streptococcal Ab) >Like VDJ recombination this involves: - enzymes (including one called activation induced cytidine deaminase) AID; - recognition of nucleotide sequences called switch (S) sequences which lie upstream of C regions - C region genes are spliced out of DNA to form circle of DNA that is eventually degraded |
|
|
|
What determines whether the Ab will be secreted or transmembrane?
|
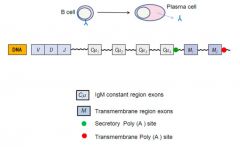
>When B cell differentiates into a plasma cell it switches from making a transmembrane version of Ab to a soluble version of Ab
- Involves non utilisation of Constant region gene encoding the transmembrane sequence |
|
|
|
In which 5 ways may TCRs diversify?
|
1. Multiple germ lines
2. V-J and V-D-J recombination 3. Recombinatorial innacuracies 4. P and N nucleotide addition 5. Chain combinations |
|
|
|
What is vaccination?
|
>Practice of Ag specific stimulation of the host immune response so as to prevent or treat infections disease.
>Modern era introduced by Edward Jenner who discovered that people could be protected from infection by smallpox virus by prior exposure to Vaccinia, a related virus. |
|
|
|
What must vaccines do in order to be effective?
|
VACCINES MUST INDUCE A RESPONSE THAT EXHIBITS:
>Memory >Specificity - memory specific to immunising antigen or closely related Ag - poses limitation for microorganisms which exhibit extensive Ag variation e.g. Influenza >Immune response must be appropriate - response stimulated by the vaccine must activate effector mechanisms which are effective against the type of organism involved |
|
|
|
What are adjuvants?
|
>Substances which boost the immune response to a co-delivered Ag by
- providing a long term depot of antigen - stimulating APCs >Common used Alum >Better ones are needed and under development |
|
|
|
What public health issues surround vaccines?
|
>Vaccines are prophylactic rather than therapeutic
>They rely on compliance rates (herd immunity) for effectiveness NB. Important to consider both factors when assessing cost/risk benefit ratios for vaccination |
|
|
|
What are examples of innovation in vaccines?
|
VACCINES:
>PCV Pneumococcal conjugate vaccine >MenC Meningococcal Group C >Cervarix vaccine agains HPV 16 and 18 DEVELOPMENT >Live recombinant vectors >DNA immunisation |
|
|
|
Which components of immunity confer protection against Extracellular bacteria; Intracellular bacteria; Viruses;
Multicellular parasites? |
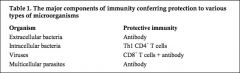
|
|
|
|
What are the 4 types of vaccine currently in use?
|
1. Live attenuated vaccine
2. Inactivated organism 3. Subunit vaccines 4. Passive immunisation |
|
|
|
Explain what is a live attenuated vaccine?
|
>Induces a strong and usually appropriate immune response
>Single dose induces long term immunity >Potential danger of reversion to virulence and cannot be used in immunocompromised individuals >Difficult to store and quality control E.g. BCG, MMR |
|
|
|
Explain what is an inactivated vaccine?
|
>Induces weaker and often inappropriate immunity; may cause some immunopathology
>No danger of reversion to virulence, maybe used in immunocompromised individuals E.g. Hepatitis A, Rabies, IPV, Influenza (although most flue vaccines contain inactivated virus, a live attenuated vaccine is also produced as a nasal spray) |
|
|
|
Explain what is a subunit vaccine?
|
>The antigen in these cases may be a toxin, viral coat protein or a bacterial cell wall component
>Approach limited by appropriateness - subunit vaccines currently in use only induce an Ab response >Subunit vaccines require multiple doses (booster) and require adjuvant addition E.g. Tetanus, diptheria, pertussis, hepatitis B, meningococcus, haemophilus |
|
|
|
Explain what is passive immunisation?
|
>Preformed antibody (made in animals or culture)
>Can be used to provide immediate temporary immunoprotection >Not really vaccination but occasionally used therapeutically post exposure E.g. Tetanus, botulism |
|
|
|
What different types of protection might be required of an aids vaccine?
|
>Prophylactic to protect against infection
>Therapeutic to prevent or delay progression to AIDS >Altruistic to prevent transmission |
|
|
|
What are the hurdles in developing an AIDS vaccine?
|
>Identification of immunogens that induce broad and long lasting CTL immunity
>Definition of structures and immunisation strategies that elicit broadly neutralising Ab |
|
|
|
What 3 components does a vaccine require?
|
>Antigen
- whole organism, subunit, bacterial toxoid >Carrier - provides Th epitopes (e.g. ovalbumin) >Adjuvant - non specifically stimulates a specific immune response |
|
|
|
What is the mechanism of DTP conjugate vaccines?
|
>Conjugates contain polysaccharide capsule with tetanus or diphtheria toxoid which converts a T-independent response to a T-dependent one.
>Why is this useful? |
|
|
|
What is meant by antigenic drift and shift?
|
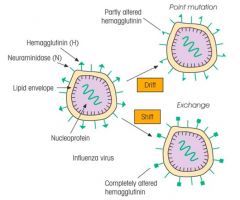
|
|
|
|
Describe the classical vaccine approaches.
|
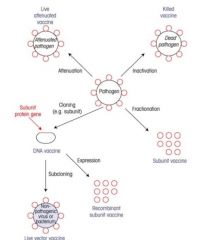
|
|
|
|
Describe the early development of haematopoiesis.
|
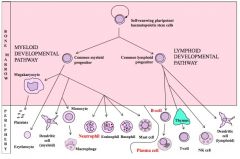
>HSCs arise in the yolk sac, then in the foetal liver
- From neonate onwards they are found in the bone marrow throughout life >Stem cells develop within a microenvironment created by distinct cell-cell interactions with - 'stromal' cells e.g. epithelial cells - guiding influence of soluble mediators such as cytokines >G-CSF induces differentiation of stem cells into granulocytes >M-CSF induces differentiation into monocytes etc. |
|
|
|
Describe the primary and secondary lymphoid organs, and what they respectively educate, and immunologically respond to.
|
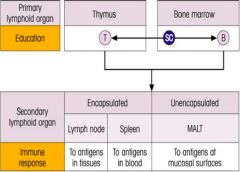
|
|
|
|
What is lymphocyte differentiation?
|
>Lymphocyte precursors undergo Ag-independent differentiation within the micro environments of the primary lymphoid organs (bone marrow and thymus)
- to become immunocompetent - then driven by Ag in the secondary lymphoid organs to develop into memory cells or effectors of the immune response |
|
|
|
What is the objective of specific immune tolerance?
|
>To prevent undesirable immune responses
- e.g. to self Ag |
|
|
|
Which arm of the immune response is involved in immune tolerance?
|
>Adaptive immune response
|
|
|
|
What occurs during B cell differentiation?
|
>Some of the bone marrow stem cells are destined to become B cells
- Undergo specific changes in expression of certain molecules - Requires IL-7 to commence differentiation 1. Express cell surface molecule CD19 and rearrange their Ag receptor (i.e. Ab) heavy chain genes 2. Cells express small amounts of Ab heavy chain in their cytoplasm (Pre B cells) 3. Subsequently rearrange one of two light chain gene loci (λ or κ) 4. Display Ab on surface where they act as Ag receptors 5. MHC I and II are also expressed on B cell surface 6. Mature B cells leave the bone marrow to function in the secondary lymphoid tissues |
|
|
|
What occurs during T-cell differentiation?
|
>Some of the bone marrow stem cells are chemotactically attracted to the thymus
- Proliferate and differentiate into T-lymphocytes as they rearrange their T-cell receptor (TCR) genes >Individually exhibit both CD4 and CD8 molecules on their surface - double positive T cells begin to express their Ag specific TCR together with CD3 molecules >Then migrate from sub-capsular outer cortex of Thymus through into the deep cortex - Here they lose expression of either CD4 or CD8 to become: - CD4+ CD8- - CD8+ CD4- >Following positive and negative selection the T cells leave the thymus and are seeded to the periphery |
|
|
|
How does the innate immune system discriminate between self and non-self antigens?
|
>Microbe PAMPs are recognised by PRRs on phagocytes
- Healthy cells are left alone because they do not express PAMPs >Ageing and apoptotic cells display damage to surface carbohydrates / loss of membrane symmetry - allows cells to be recognised and phagocytosed >Complement system not normally triggered by our own cells because of complement inhibitory molecules on their surface. |
|
|
|
How does the innate immune system discriminate between self and non-self antigens?
|
>Antigen specific TCR and BCRs could potentially recognise self antigens (same amino acids used to build them)
>Lymphocytes however are unable to respond to self antigens and we are unresponsive or 'tolerant' of our own antigens >Tolerance occurs at central and peripheral levels |
|
|
|
Explain the structure and role of the thymus.
|
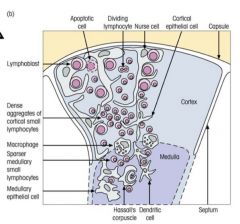
FUNCTIONS:
>To produce many different T cells with receptors that recognise foreign Ag (generating receptor diversity) >Select T cells so that they can recognise the body's own MHC molecules (positive selection) >To eliminate those T cells with Ag receptors reacting with self Ag (negative selection) |
|
|
|
What is the difference between parenchyma and stroma?
|
>The parenchyma of an organ consists of that tissue which conducts the specific function of the organ and which usually comprises the bulk of the organ.
>Stroma is everything else -- connective tissue, blood vessels, nerves, ducts. The parenchyma / stroma distinction provides a convenient way to circumvent the listing of tissue types when discussing an organ. Examples: >The parenchyma of the kidney is epithelial tissue (renal tubules and corpuscles). - The blood vessels, nerves, and supporting connective tissue of the kidney comprise the stroma. >The parenchyma of the spleen is connective tissue (mostly lymphocytes and other blood cells). - The supporting fibrous connective tissue of the spleen comprises the stroma. >The parenchyma of the heart is muscle tissue (cardiac muscle cells). - The nerves, intrinsic blood vessels, and connective tissue of the heart comprise the stroma. >The parenchyma of the brain is nervous tissue (nerve cells and glia). - The blood vessels within the brain and the connective tissue associated with these blood vessels are stroma. >The parenchyma of the malignant neoplasm is cancer cells. - Other tissues, including blood vessels, which grow to support the tumor are stroma. NB. >Parenchyma is interesting. Because organ-specific function usually centres on parenchymal cells, histological (and physiological) accounts often emphasize parenchyma. >Unfortunately, stroma is commonly ignored as just boring background tissue. Pay attention to the stroma. No organ can function without the mechanical and nutritional support provided by the stroma. If an organ is inflamed, the signs of inflammation appear first in the stroma. |
|
|
|
Explain what is meant by central tolerance.
|
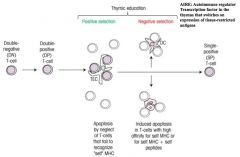
>Central tolerance results from the elimination of self-reactive cells during lymphocyte development
>TCRs recognise processed antigens bound to cell surface MHC molecules - Part of TCR recognises foreign peptide - Part recognises the self MHC molecule >Random nature of TCR gene rearrangements means that only a minority of T cells are capable of performing this task - Many immature CD4 and CD8 double +ve T cells are useless because their TCRs do not recognise self MHC molecules at all :. undergo apoptosis >Cells whose TCRs have various affinities for binding self MHC molecules (usually continuing a self peptide) are first positively selected on thymic CORTICAL EPITHELIAL cells - many of these are potentially harmful because their TCRs have a high affinity for complex of self-peptide and self-MHC molecule - These cells are eliminated by the induction of apoptosis when they interact with dendritic cells and macrophages in the thymic medulla (negative selection) >Negative selection of T cells in the thymus leaves T cells with only a weak affinity for self MHC molecules - These cells form the pool of T lymphocytes that are exported from the thymus as single positive (CD4 or CD8) cells - In the periphery they have the potential to recognise a complex of foreign peptide plus self-MHC molecules and to become activated if the affinity of the interaction exceeds a certain threshold |
|
|
|
What is AIRE?
|
Autoimmune regulator transcription factor in the thymus that switches on expression of tissue restricted Ag.
|
|
|
|
What are the implications of lack or faulty Th cells?
|
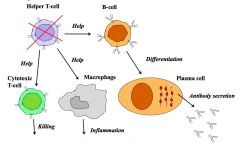
|
|
|
|
Explain what is meant by peripheral tolerance?
|
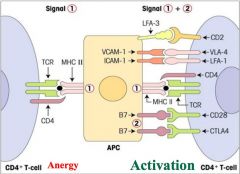
>Peripheral i.e. outside of Thymus and Bone marrow
>Prevents harm from any self-reactive cells that manage to escape deletion in the primary lymphoid organs - Such escape can occur because not all self-Ag are present in the primary lymphoid organs >Lymphocytes in the periphery are normally kept in an unresponsive state through clonal anergy - Cells don't receive the pathogen triggered costimulatory signals necessary for activation of the cell - autoreactive lymphocytes can also be suppressed by regulatory T-cells NB. Breakdown in tolerance to self antigens can lead to autoimmune disease. |
|
|
|
What is the difference between nTreg and iTregs? What is their role?
|
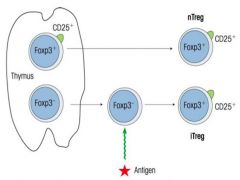
>Suppression of auto reactive lymphocytes
|
|
|
|
How do Tregs suppress autoreactive lymphocytes?
|
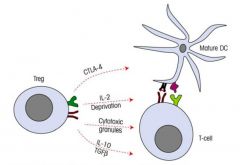
|
|
|
|
What is immunological tolerance?
|
>Property of lymphocytes
>T and B cells become tolerised - Via clonal deletion or anergy - Suppression >Prevents harmful immunity |
|

