![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
111 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Innate Immunity: Built in from birth? Antigen specific? Uses cellular and humoral (soluble) components? How quick? |
|
|
|
|
Which cells are exclusive to the adaptive immune system? |
Lymphocytes & Antibodies |
|
|
|
Which cells are exclusive to the adaptive innate system? |
Neutrophils & NK cells |
ALL Ns |
|
|
Which cells are exclusive to the humeral and cells of the immune system? |
Monocytes, Macrophages, Dendritic cells, Mast cells, complements, and cytokines. |
MMMDCC |
|
|
Innate immune recognition strategies, give an example of each |
1. Detect conserved microbial structures - PAMPs, ds DNA 2. DAMPs, Extracellular ATP 3. Detect 'self', using MHC class I specific receptors |
|
|
|
Describe the feature of a neutrophil |
|
|
|
|
Neutrophils fighting sequence? |
1. Migrate to site of infection (Diapedesis and Chemotaxis). 2. Bind pathogen- Opsonisation. 3. Phagocytose 4. Kill pathogen |
4 steps |
|
|
How do neutrophils kill pathogen done without oxygen via |
|
|
|
|
How do neutrophils kill pathogen done without oxygen via |
|
|
|
|
Chemotaxis: |
1. Chemokines are released which bind to the local endothelial layer 2. Chemokines are released which bind to the local endothelial layer 3. Chemokines are released which bind to the local endothelial layer 4. Chemokines are released which bind to the local endothelial layer |
|
|
|
What are the two opsonins? |
|
|
|
|
How is neutrophil phagocytosis made more efficient |
This is much more effective after opsonisation (coating of the pathogen to make it easier for the neutrophils to recognise the pathogen). |
|
|
|
Cytokines: |
|
|
|
|
Types of Cytokine |
|
|
|
|
Cytokine transmission |
Autocrine Action Paracrine Action Endocrine action |
|
|
|
Important Cytokines |
IL-1alarm cytokine fever TNF-alarm cytokine IL-6 acute phase proteinsliver IL-8 chemotactic for neutrophils IL-12 directs adaptive immunityactivates NK cells |
|
|
|
Bacterial Septic Shock |
Systemic infection massive release of the TNF- and IL-1 by activated macrophages Increased vascular permeability Sever drop in blood pressure which results in10% mortality |
|
|
|
Dendritic Cells |
|
|
|
|
Complement. Role? Amount? Speed? Produced where? |
|
“describe the activity in serum which could complement the ability of specific antibody to cause lysis of bacteria” Ehrlich (1854-1915) |
|
|
Complement Activation THREE PATHWAYS: |
Classical Alternative Lectin Pathway |
|
|
|
Classical pathway |
Antigen binding to antibody causes aconformational change – activates complement |
|
|
|
Alternative Pathway |
Direct contact with thepathogen surface activates complement |
|
|
|
Lectin pathway |
Activated by lectin (which is aPRR) binding to carbohydrates that are only found on pathogens |
|
|
|
Describe where and how the three pathways converge |
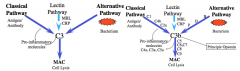
|
|
|
|
What happens to the cleaved fragments during thecomplement cascade? |
They are pro-inflammatory molecules, which can bindto receptors on mast cells and cause degranulation giving rise to an inflammatory response. |
|
|
|
Complement is controlled: |
1. lability of components, i.e. their short half-life 2. Dilution of components in biological fluids 3. Specific regulatory proteins e.g Factor H |
3 things |
|
|
What is the function of the Complement? |
1. chemotaxis 2. Opsonisation 3. Inflammation 4. Lysis: Activation of C3 is a common final pathway which leads to the formation of MEMBRANE ATTACK COMPLEX (MAC) which is what lyses infected cells or bacteria. |
COIL |
|
|
Mast Cells function? |
|
|
|
|
Summarise a typical inflammatory response to a localised infection involving recruitment of neutrophils, and phagocytosis and killing bacteria |
|
AlarmDirectComplementDegranulate |
|
|
Which compliment protein Mast cells are activated by? |
anaphylatoxins |
|
|
|
Briefly summarise the events involved in a systemic acute phase response |
|
|
|
|
What acute phase proteins are involved in the systemicacute phase response? |
C-reactive protein (CRP), mannan-binding lectin, fibrinogenand complement |
Creative man fibres complement |
|
|
Natural Killer (NK) Cells Size? Function? Interferon? Antigen Specific? Receptors? |
|
|
|
|
Summarise the phenotype and functions of natural killer (NK) cells |
|
|
|
|
Target Cell Recognition: Missing Self Recognition |
An infected,cells will downregulate the expression of MHC Class I, which acts as an inhibitory signal. The loss of the inhibitory signal means that NK cells are more likely to killthe target cells. |
|
|
|
Antibody Definition |
a protein that is produced in response to a foreign molecule (antigen), and has the property of binding specifically to that antigen. |
|
|
|
What produces Antibodies |
Antibodies are produced by B-‐‑lymphocytes |
|
|
|
What are the three secondary effector functions of antibodies once bound to antigens? |
Opsonisation Complement activation Cell activation via specific antibody-binding receptors (Fc receptors) |
|
|
|
Structure: Antibody |
|
|
|
|
The discovery of antibody structure? |
Limited the digestion of gamma-globulin with purified papain, which produced 3 fragments in equal amounts 2 fragments had antigen binding activity (Fab). The third did not, but formed protein crystals (Fc) |
|
|
|
What type of bond holds together the chains in theimmunoglobulin? |
Disulphide bonds |
|
|
|
Describe the constant and variable region of the light and heavy chains? |
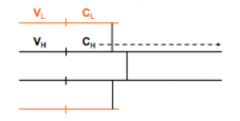
Both light and heavy chains can be divided into variable (where the sequences are different) and constant (same sequence) regions |
|
|
|
Function of the Fc part? |
The Fc part changes conformation when the antigen is bound and can perform effector functions such as activating complement. |
|
|
|
Define cross-reactivity when it comes to the properties of antigen binding site? |
Antibodies are highly specific but they will sometimes react with other molecules. There are 3 regions which are hyper variable, called CDR |
|
|
|
Describe the antigen binding site |
Antigen binding occurs at 3 HYPERVARIABLE regions, known as COMPLEMENTARITY DETERMINING REGIONS (CDR’s). these loops of amino acids are the part of the protein that is actually binding to the antigen and determines the specificities. |
|
|
|
Which forces involved when binding |
Hydrogen bonds Ionic bonds Hydrophobic interactions Van der Waals interactions |
NON-COVALENT |
|
|
What part of the variable region of the antibody binds to the antigen? |
The complementarity determining regions are found atthe end of the variable regions and interact with antigens |
|
|
|
Define: Afinity in terms of antibodies |
The strength of the total non-covalent interactions between a single antigen-binding site and a single epitope on an antigen. |
|
|
|
Define: Avidity in terms of antibodies |
The overall strength of multiple interactions between an antibody with multiple binding sites and a complex antigen with multiple epitopes. |
|
|
|
Avidity or Affinity? Which is a better tool of measuring
|
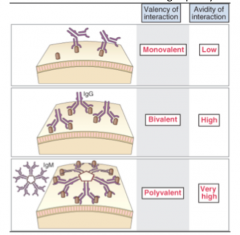
Avidity, because antibodies have multiple binding sites and form a complex with multiple epitopes |
|
|
|
What is antibody cross-reactivity? Give an example. |
Antibodies that are produced in response to one antigen can cross-react and bind to a different antigen with a similar structure. E.g. cow pox and small pox ABO blood types |
|
|
|
Which region do of the antibody determines its class? |
Different classes of antibodies differ in the constant regions of their heavy chains |
|
|
|
Which two Ig classes are mainly responsible for activating complement? |
IgM and IgG |
|
|
|
The generic Class: HC: HC domain: LC: |
Class: IGx Heavy Chain: x in greek Heavy Chain domains: 3 for all, bar IgM & IgE Light Chain: either kappa or lambda |
|
|
|
Which Ig have subclasses and how many? |
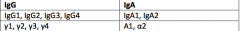
FOUR IgG subclasses - 1 to 4 TWO IgA subclasses - 1 to 2 |
|
|
|
IgG |
|
SCAN P |
|
|
IgA |
|
SSMMJ |
|
|
There is a process to get the dimeric IgA from below the epithelial layer into the lumen. What is it? |
|
|
|
|
IgM |
|
P P A A |
|
|
IgD |
|
IgD more like B |
|
|
IgE |
|
all E rgy
|
|
|
How to remember the Igs? |
GMEAD 1*BSB |
|
|
|
Where are Ig's found? |
Blood = IgG + IgM Extracellular Fluid = IgG Secretions across epithelia (inc. breast milk) = Dimeric IgA Foetus = IgG Mast Cells below Epithelia= IgE |
|
|
|
There is a key difference between T cells and B cells in the TYPE OF EPITOPE they recognise: |
|
|
|
|
Explain the origin and maturation of B lymphocytes |
|
|
|
|
what determines the class of anti-body |
The constant region is what determines the type of antibody e.g. alpha constant region gene gives rise to IgA |
|
|
|
Describe the BCR structure and how it drives an intracellular signal |
|
|
|
|
Describe the properties of the B cell Receptor |
|
|
|
|
What is the process by which B cells and T cellsgenerate the variety in their receptors/antibodies? |
Immunoglobulin Gene Rearrangement |
|
|
|
Describe the generation of variation in the lightchain. |
1. There are 70 different V and J regions 2. The B cell begins with germline DNA and it cuts V and J regions at randomly, meaning that there is a large number of different combinations 3. Different splicing patterns give rise to more variation 4. |
|
|
|
Describe the generation of variation in the heavy. |
Gene rearrangement is the same – the only differenceis that the heavy chain also has a D region and has several different constantregions (determines class) |
|
|
|
Where are the BCR genes located? |
Each BCR chain (κ & λ light chains, and heavy chain) is encoded by separate MULTIGENE FAMILIES ON DIFFERENT CHROMOSOMES |
|
|
|
Describe the generation of variation in the heavy chain. |
1. The same as the light chain. 2. ALTERNATIVE SPLICING; results in different mature mRNA, as the mRNA express different genes (e.g. they may have different constant region genes present) |
|
|
|
What enzyme is involved in the removal of unusedsegments of DNA? |
V(D)J Recombinase |
|
|
|
What determines the class of the immunoglobulin? |
The constant region of the heavy chain |
|
|
|
Selection for self tolerance? |
checking that not made antigen against itself |
|
|
|
In what order does the gene rearrangement take place? |
Heavy Chain undergoes VDJ rearrangement FIRST, Light chain then undergoes VJ rearrangement |
|
|
|
What three things can happen to B cells once they'verecognised their antigens? |
IN THE LYMPHNODE: Affinity Maturation Become Memory cells Become Plasma cells (antibody production) |
|
|
|
What is the general rule about B cell and T cellactivation? |
It needs co-stimulation to be activated – antigen alone is not enough. From either the Directly from microbial constituents T helper cell |
|
|
|
What are the two pathways by which B cell productionis achieved? |
T cell dependent: T cell independent: This is why people with Di-Geogre syndrome have antibodies |
|
|
|
Describe the T independent pathway. |
1. Main thing it's related to is polysaccharides 2. The polysaccharide has a repeating subunit because the molecule is long and repeating it binds to lots of BCR on the same cell and drive cross-linking 3. One molecule will be recognised by lots of different receptors and pulled into the same space. 4. You also need a secondary signal In T independent antigens, the secondary signal is coming from microbial constituents - PAMPs such as LPS. |
|
|
|
Describe the T dependent pathway. |
1. The antigen has to be taken up by TWO TYPES OF CELL - B cell + Dendritic Cell. 2. The antigen is chopped up and put on the MHC class II - ON BOTH CELL - this is what allows APCs to interact with T cells. 3. The loaded MHC class II is presented to a T cell which recognises it through TCR 4. In the lymph nodes they bind to the B cell which has the same MHC class II with antigen. THIS PROVIDES THE SECOND SIGNAL The B cell becomes a plasma cell |
|
|
|
How do T cells activate B cells? |
|
|
|
|
Describe the process of immunoglobulin classswitching. |
1. Once the B cells are in contact with the T cell - the T cell drives the class switching 2. You keep the variable region and you switch out different exons to give you a different constant region 3. Certain cytokines help to produce certain Ig classes during differentiation of CENTROCYTES into plasma cells |
constant region |
|
|
What drives the improvement of the immune response between primary and secondary exposures? |
|
|
|
|
Immunological Memory |
|
|
|
|
When B cells go bad? |
|
|
|
|
T lymphocytes |
Destroy intracellular pathogens T cell receptor (TCR) recognizes small peptide fragment of antigen presented by MHC molecule on the surface of host infected cell |
|
|
|
Describe: T cell receptor (TCR) |
|
|
|
|
How does the TCR communicate intracellularly when it binds to an antigen? |
1. CD3 receptors have much longer tails with motifs containing tyrosine residues 2. so when the TCR meets its antigen, PHOSPHORYLATION OF TYROSINE in the motifs occurs. 3. CD3 is important in sending signals to the lymphocyte and it is useful as a marker because it is present on all T lymphocytes |
|
|
|
Which T cell is the, Antigen Recognition: CD4 |
|
|
|
|
Which T cell is the, Antigen Recognition: CD8 |
|
|
|
|
CD3 function |
CD3 is important in sending signals to the lymphocyte and it is useful as a marker because it is present on all T lymphocytes |
|
|
|
CD4 - T helper cells function |
|
|
|
|
CD8 - Cytotoxic T Lymphocytes |
Kill target cells Induce apoptosis in target cells |
|
|
|
CD4+ Th1 |
Involved in inflammatory responses Activates the macrophages so they can kill phagocytosed material |
|
|
|
CD4+ Th2 |
Important in helping B cell response. Th2 binds and activates the B cell so it starts producing a response |
|
|
|
Describe, in full, T cell development in the thymus. |
1. In the cortex of the thymus, precursor cells arrive with no TCR, CD4, CD8 receptors. 2. Germline DNA undergoes recombination and rearrangement. 3. Beta chain is rearranged FIRST, then alpha chains. 4. Then CD4 and CD8 are expressed. 5.Depending on which type of MHC they recognise, they will either become CD4+ or CD8+ |
|
|
|
The TCR checkpoints |
For 1-4, if no, then apoptosis |
|
|
|
Selection: T cells Post TCR checkpoint |
Post TCR checkpoint - Is the αβ TCR functional? - Is the αβ TCR dangerous/autoreactive? - Useless: cannot see MHC – die by apoptosis - Dangerous: see “self”, i.e. host molecules – receive signal to die by apoptosis |
|
|
|
MCH 1 STRUCTURE |
|
|
|
|
MHC 2 STRUCTURE |
|
|
|
|
MHC 1 GEOMETRY |
|
|
|
|
MHC 2 GEOMETRY |
|
|
|
|
Define Human Leukocyte Antigen, and when/where they are expressed? |
The polypeptides that make up the MHC molecules are encoded within the HLA region. MHC Class I: present in nearly all nucleated cells, and levels may be altered during infection or by cytokines MHC Class II: normally only on professional APC, and may be regulated by cytokines |
|
|
|
Describe MHC gene expression |
|
|
|
|
Is MHC polymorphic? |
|
|
|
|
What is the difference between the types of peptides presented by Class I and Class II? |
Class I presents peptides that are smaller than theMHC moleculeClass II presents peptides that are longer than theMHC molecule so it often has bits protruding out of the MHC molecule |
|
|
|
Describe the process of antigen presentation via MHC Class I? |
1. Antigen cleaved by proteasome, taken into RER by TAP (transporter associated with antigen presenting) - 2. Bind with MHC class I - Shaperones, e.g. calnexin, help protein 3. Then trafficked by golgi to surface |
|
|
|
Describe antigen presentation via MHC Class II. |
1.Antigen endocytosed 2. Cleaved by proteases 3. MHC II migrates into RER- associates with INVARIANT chain 4. The MHC II –invariant complex is migrated into the golgi in ENDOSOME 5. Invariant chain is digested by CLIP (Class II associated invariant chain peptide) 6. CLIP is then exchanged for the antigenic peptide, which is then presented at the surface |
|
|
|
Why is it that such a variety of peptides can bind to the MHC, yet it still be specific? |
MHC presents a subset of peptides which have some things in common where characteristics are conserved. |
|

