![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
70 Cards in this Set
- Front
- Back
|
Response to an initial infection occurs in 3 phases. Explain
|
1. Innate Immunity (immediate, 0-4hrs)
* infection >> recognition by nonspecific and broadly specific effectors >> removal of infectious agent 2. Early Induced Innate response (early, 4-96hrs) ** infection >> recognition of microbial-associated molecular patterns >> inflammation recruitment and activation of effector cells >> removal of infectious agent 3. Adaptive Immune Response (late, 96+hrs) *** infection >> transport of antigen to lymphoid organs >> recognition by naive B and T cells >> clonal expansion and differentiation to effector cells >> removal of infectious agent |
|
|
Pathogenensis?
|
-- means by which it causes disease and the response it elicits from the host
|
|
|
zoonotic infection
|
-- infections of humans from animal reservoirs
|
|
|
what if mucosal epithelia?
|
-- internal epithelia that secrete viscous fluid called mucous (which contains glycoproteins called mucins)
|
|
|
An infection and the response can be divided into series of stages
|
1. Adherence to epithelium
>>Protection agains infection by: --normal flora --local chemical factors --phagocytes (esp.in lungs) 2. Local infection that breaks skin (penetrate epithelium) >>protection against infection: --wound healing induced antimicrobial proteins+peptides --phagocytes+complement destroy invading microorganisms --activation of γ or δ T-cells 3. Local infection of Tissues >>protection against infection: --complement, cytokines, chemokines, phagocytes, NK cells --activation of macrophages --dendritic cells migrate to lymph nodes to initiate adaptive immunity --blood clotting helps limit spread of infection 4. Adaptive Immunity >>protection against infection: --infection cleared by specific antibody --Tcell dependent macrophage activation --cytotoxic Tcells (Tc) |
|
|
Who are the phagocytes and what are their functions?
(the next line of defense after the surface epithelia) |
1. macrophages
>> phagocytosis and activation of bactericidal mechanism >> antigen presentation 2. dendritic cell >> antigen uptake in peripheral sites >> antigen presentation in lymph nodes 3. neutrophil >> phagocytosis and activation of bactericidal mechanism |
|
|
Phagocytes use receptors to increase efficiency
|
1. macrophages express receptors for many bacteria constituents: mannose receptors, LPS receptors (CD14), glucan receptor, scavenger receptor, and TLR-4 (toll-like receptor)
2. bacteria binding to macrophage receptors to initiate release of cytokines and small lipid mediators of inflammation 3. macrophage engulf and digest bacteria that they bind to. |
|
|
Class of mechanism of phagocytes and bactericidal agents they produce
|
1. acidification mechanism: pH between 3.5-4 (bacteriostatic/ bactericidal)
2. toxic, O2-derived products: superoxide, hydrogen peroxide, singlet oxygen, hydroxyl radical 3. toxic nitrogen oxides: nitric oxide 4. antimicrobial peptides: cationic proteins and defensins 5. enzymes: --lysosomes dissolve walls of gram pos.bacteria --acid hydrolases: further digest bacteria 6. competitors: lactoferrin (binds Fe) and vitamin B12 binding protein |
|
|
Inflammation in a nutshell
|
* the way we send signals throughout the body to make it less hospitable for pathogens
* to recruit more immune cells to site of infection to kill more pathogens * activate phagocytic cells (macrophages) * initiated by damaged epithelial cells and active phagocytic cells |
|
|
Classical Characteristics of Inflammation
|
1. pain (release of kinins)-- biological effect to direct attention to site of injury
2. swelling (increased vascular permeability, adhesion) 3. heat (increased vascular diameter) 4. redness (increased vascular diameter) |
|
|
what are the 3 types of changes locally occur in blood vessel?
|
1. increased in vascular diameter (vasodilation)
2. expression of adhesion molecules 3. increased in vascular permeability |
|
|
What are 3 important roles that inflammation plays?
|
1. recruitment of effector cells to site of infection
2. provide physical barrier (local blood clotting) to prevent spread of infection 3. promote repair of injured tissues |
|
|
Cytokines vs Chemokines
|
1. CYTOKINES are
-- secreted by activated macrophages -- small proteins made by cells (macrophages) that affect the behavior of other cells by binding with specific receptors (signaling messenger to other cell) * LYMPHOKINES are cytokines made by lymphocytes * INTERLEUKINES are cytokines released by leukocytes and affect other leukocytes 2. CHEMOKINES: -- cytokines with chemo-attactive properties (stimulate migration and activation of cells) -- can recruit other cells to site of infection |
|
|
what are the different kinds of cytokines that activated macrophages secrete?
|
Hematopoetin family:
* IL-1 β * IL-6 TNF family: * TNF-α * CXCL-8 Structurally distinct: * IL-1 * IL-12 |
|
|
what are the local effects and systemic effects of IL-1 β?
|
Local:
-- activates vascular endothelium -- activates lymphocytes --local tissue destruction --increases access of effector cells |
|
|
what are local and systemic effects of TNF-α?
|
Local Effects
-- activates vascular endothelium and increases vascular permeability, leading to increased entry of IgG, complement and cells to tissues -- increased fluid drainage to lymph nodes Systemic Effects -- fever -- mobilization of metabolites -- shock |
|
|
what are local and systemic effects of IL-6?
|
Local
-- lymphoid activation -- increased antibody production Systemic Effect -- fever -- induces acute-phase protein production |
|
|
what are local effects of CXCL-8 and IL-12?
|
CXCL-8:
-- chemotactic factor recruits neutrophils, basophils, and T-cells to site of infection IL-12: -- activates NK cells -- induces the differentiation of CD4 T-cells into Th1 cells |
|
|
Overview of Complement Cascade (Brief)
|
1. Classical Pathway:
antigen-antibody complexes 2. Lectin Pathway: lectin binding to pathogen surfaces 3. Alternative Pathway: pathogen surfaces >>combined lead to COMPLEMENT ACTIVATION 1. Classical Pathway >> RECRUITMENT of inflammatory and immunocompetent cells 2. Lectin Pathway >> OPSONIZATION of pathogens 3. Alternative Pathway >> KILLING of pathogens |
|
|
what does complement mean?
|
a series of proteins synthesized as inactive proteins but activated by protein activation cascades to function.
** initial activation will lad to activation of other proteins |
|
|
3 activation pathways (of complement cascade) -- part I
|
1. CLASSICAL pathway
-- antigen-antibody complexes (pathogen surface) -- proteins: C1q, C1r, C1s, C4, C2 2. MB-Lectin Pathway: -- mannon binding lectin binds mannose on pathogen surfaces -- proteins: MBL, MASP-1, MASP-2, C2, C4 3. Alternative Pathway -- pathogen surfaces -- proteins: C3, B, D *** ALL pathways lead to C3 CONVERTASE (activate C3) *** activation can happen simultaneously *** Opsonization: coating of something (pathogen) for easier phagocytosis |
|
|
once activation occurs (complement cascade part II)
|
After C-3 Convertase:
1. Classical Pathway: -- lead to C4a, peptide mediators of inflammation and phagocyte recruitment 2. Lectin Path: -- C3b binds to complement receptors on phagocytes -- Opsonization of pathogen and removal of immune complexes 3. Alternative Path: -- terminal complement components (C5b, C6, C7, C8, C9) -- lead to membrane attack complex, lysis of certain pathogens and cells |
|
|
what is MAC
|
Membrane Attack Complex
=deposited on pathogen that will "ok" the attack on pathogen |
|
|
Classical Pathway (of complement cascade), steps:
|
* activated C1 cleaves C4 to a C4a and C4b, which bind to microbial surface
* C4b binds C2, which is cleaved by C1s to C2a and C2b, forming C4b2a complex * C4b2a is an active C3 convertase cleaving C3 to a C3a and C3b which binds to microbial surface or to the convertase itself * one molecule of C4b2a can cleave up to 1000molecules of C3 to C3b (many of which bind to microbial surface) |
|
|
Alternative Pathway amplifies other pathways
|
* C3b deposited by classical/ lectin path C3 convertase
* C3b binds factor B * Bound factor B is cleaved by plasma protease factor D into Ba and Bb * C3bBb complex is a C3 convertase, cleaving many C3 molecules to C3a and C3b * can amplify the classical/ lectin path by forming an alternative C3 convertase an depositing more C3b molecules on pathogen |
|
|
3 main classes of innate-like lymphocytes and their properties
|
1. B-1 cells:
--make natural antibody --ligands not MHC associated --cannot be boosted 2. Epithelial γ and δ cells: --produces cytokines rapidly --ligands are MHC class IB associated --cannot be boosted 3. NK T-cells: --produce cytokines rapidly --ligands are lipid bound to CD1d --cannot be boosted |
|
|
what are the major activating receptors of NK cells?
|
* NKp30
* NKp40 * NKp46 * NKG2D |
|
|
what are the 4 major functions of interferons IFN-alpha and IFN-beta?
|
1. they induce resistance to viral replication in all cells
2. increase MHC I expression and antigen presentation in all cells 3. activate dendritic cells and macrophages 4. activate NK cells to kill virus-infected cells |
|
|
what are the difference between release of TNF-alpha locally and systemically?
|
1. LOCAL infection w.gram neg.bacteria:
-- activates macrophages to secrete TNF-alpha in the tissue --increased release of plasma proteins into tissue, increased phagocyte and lymphocyte migration into tissue, increased platelet adhesion to BV walls --phagocytosis of bacteria; local vessel occlusion. Plasma and cells drain to local lymph nodes -- removal of infection (adaptive immunity) 2. SYSTEMIC infection with gram neg.bacteria: --macrophages activated in liver and spleen secrete TNF-alpha into bloodstream --causes systemic edema, causing decrease Bvol, leading to vesels collapse --disseminated of intravascular coagulation leading to wasting and multiple organ failure -- lead to death |
|
|
the alternative pathway (part I)
|
* alt path doesn't need signaling
* C3 undergoes spontaneous hydrolysis to C3(H20) which binds to factorB allowing it to be cleaved by factorD into Ba and Bb * the C2(H20)Bb complex is an C3 convertase cleaving more C3 into C3a and C3b. C3b is rapidly inactivated unless it binds to cell surface * factorB binds non-covalently to C3b on a cell surface and is cleaved to Bb by factorD |
|
|
Outcomes of alternative path
(alternative path part II) |
1. on host cells, complement-regulatory proteins CR1, H, MCP, and DAF bind to C3b. Cr1, H, and DAF displace Bb
--C3 bound to H, CR1 and MCP is cleaved by factorI to yield inactive C3b (iC3b) >>NO ACTIVATION of complement on host cell surfaces 2. pathogens lack complement regulatory proteins. Binding of properdin (factorP) stabilize the C3bBb complex --- C3bBb complex is a C3 convertase and deposits many molecules of C3b on pathogen surface >> OPSONIZATION activation of terminal complement component |
|
|
T-cell activation (from a naive Tcell)
|
***Requires BOTH antigen and co-stimulatory signals:
1. no antigen >> no antigenic peptide, no response 2. no co-stimulation (foreign antibody >> no activation, Tcell becomes unresponsive 3. Both antigen and co-stimulation >> Tcell activation |
|
|
what are the Poly Morphonuclear Neutrophilic Lymphocytes (PMNs)
|
-- second major family of phagocytes (neutrophils)
-- short lived cells, abundant in the blood but not present in normal, healthy tissues |
|
|
what are mononuclear phagocytes?
|
-- phagocytes (macrophages that mature continuously from monocyte and leave circulation into tissues in the body)
-- found in large numbers in the connective tissues, submucosal layer of GI tract, and in the lung ie: Kupffer cells (in liver) |
|
|
what are the proteins of classical pathway?
|
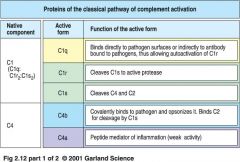
classical pathway proteins
|
|
|
what are the proteins of classical pathway (part II)
|
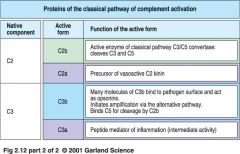
classical pathway proteins (part II)
|
|
|
what are the proteins of classical pathway?
|
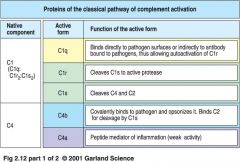
classical pathway proteins
|
|
|
the alternative pathway (part I)
|
* alt path doesn't need signaling
* C3 undergoes spontaneous hydrolysis to C3(H20) which binds to factorB allowing it to be cleaved by factorD into Ba and Bb * the C2(H20)Bb complex is an C3 convertase cleaving more C3 into C3a and C3b. C3b is rapidly inactivated unless it binds to cell surface * factorB binds non-covalently to C3b on a cell surface and is cleaved to Bb by factorD |
|
|
Outcomes of alternative path
(alternative path part II) |
1. on host cells, complement-regulatory proteins CR1, H, MCP, and DAF bind to C3b. Cr1, H, and DAF displace Bb
--C3 bound to H, CR1 and MCP is cleaved by factorI to yield inactive C3b (iC3b) >>NO ACTIVATION of complement on host cell surfaces 2. pathogens lack complement regulatory proteins. Binding of properdin (factorP) stabilize the C3bBb complex --- C3bBb complex is a C3 convertase and deposits many molecules of C3b on pathogen surface >> OPSONIZATION activation of terminal complement component |
|
|
T-cell activation (from a naive Tcell)
|
***Requires BOTH antigen and co-stimulatory signals:
1. no antigen >> no antigenic peptide, no response 2. no co-stimulation (foreign antibody >> no activation, Tcell becomes unresponsive 3. Both antigen and co-stimulation >> Tcell activation |
|
|
what are the Poly Morphonuclear Neutrophilic Lymphocytes (PMNs)
|
-- second major family of phagocytes (neutrophils)
-- short lived cells, abundant in the blood but not present in normal, healthy tissues |
|
|
what are mononuclear phagocytes?
|
-- phagocytes (macrophages that mature continuously from monocyte and leave circulation into tissues in the body)
-- found in large numbers in the connective tissues, submucosal layer of GI tract, and in the lung ie: Kupffer cells (in liver) |
|
|
phagosome
|
= endocyctic vacuole (membrane enclosed vesicles), that contain acid to kill pathogen (feature of a phagocyte)
|
|
|
what causes respiratory burst in macrophages and neutrophils?
|
a transient increase in O2 consumption during production of microbial O2 metabolites
** respiratory burst generates Superoxide |
|
|
what is a C1
|
* the first protein involved in complement cascade activation
* is a complex of C1q, C1r, C1s |
|
|
how does C1 actually work?
|
1. pentameric IgG molecules bind to antigen on bacterial surface and adapt a staple form
>> C1q binds to a bound IgM molecule 2. IgG molecule bind to antigens on bacterial surface >> C1q binds to at least 2 of IgG molecules **Both step 1 and 2 lead to binding of C1q to Ig activates C1r which cleaves and activates serine protease C1s |
|
|
what is MASP1 and MASP2?
|
* the first proteins involved in MB-Lectin pathway
* binding to mannose found on surface of pathogens, activates MASP1 and MASP2 * activated MASP2 cleaves C4 |
|
|
phagosome
|
= endocyctic vacuole (membrane enclosed vesicles), that contain acid to kill pathogen (feature of a phagocyte)
|
|
|
what causes respiratory burst in macrophages and neutrophils?
|
a transient increase in O2 consumption during production of microbial O2 metabolites
** respiratory burst generates Superoxide |
|
|
what is a C1
|
* the first protein involved in complement cascade activation
* is a complex of C1q, C1r, C1s |
|
|
how does C1 actually work?
|
1. pentameric IgG molecules bind to antigen on bacterial surface and adapt a staple form
>> C1q binds to a bound IgM molecule 2. IgG molecule bind to antigens on bacterial surface >> C1q binds to at least 2 of IgG molecules **Both step 1 and 2 lead to binding of C1q to Ig activates C1r which cleaves and activates serine protease C1s |
|
|
what is MASP1 and MASP2?
|
* the first proteins involved in MB-Lectin pathway
* binding to mannose found on surface of pathogens, activates MASP1 and MASP2 * activated MASP2 cleaves C4 |
|
|
what are proteins involved in alternative pathway?
|
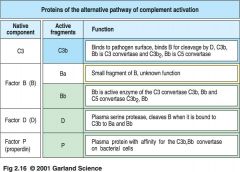
alternative pathway proteins
|
|
|
Activation pathways Comparison
|
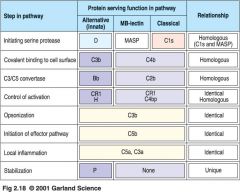
which activation pathway?
|
|
|
what are the proteins of Membrane Attack Complex (MAC)?
|
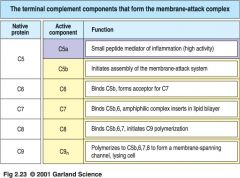
MAC proteins
|
|
|
what are the the receptors for the complement proteins?
|
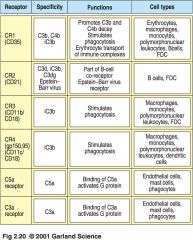
Complement Protein Receptors
|
|
|
what are the proteins that regulate complement activity?
|
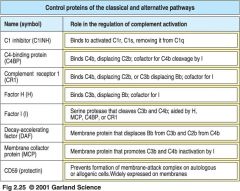
Proteins that regulate complement activity
|
|
|
Generation of C5 convertase
|
1. C3b binds to both C4b2a and C3bBb, forming active C5 convertases C4b2a3b and C3b2Bb
2. C5 binds to C3b component of the C5 convertase enzyme 3. C5 is cleaved by C2a or Bb to form C5b or C5a |
|
|
anaphylotoxin C5a enhances phagocytosis
|
1. bacterium is coated with complement by the alternative and MBL pathways
2. When only C3b binds to CR1, bacteria are not phagocytosed 3. C5 can activate macrophages to phagocytose via CR1 |
|
|
what are the other anaphylotoxins?
|
*small components of C3a, C4a and C5a when produced in large amounts or injected systemically, can induce generalized circulatory collapse (like a systemic allergic reaction involving antibody of the IgE class)
|
|
|
what are local inflammation induced by complement fragments?
|
1. small complement-cleavage products (C3a, C5a and C4a) act on BVs to increase vascular permeability and cell-adhesion molecules
2. increased permeability allows increase fluid leakage from BVs and let out Ig (IgG and IgM) and complement molecules 3. migration of macrophages, PMNs and lymphocytes is increased. Microbicidal activity of macrophages and PMNs is also increased. |
|
|
Stages where complement activity id regulated
|
1. C1q binding to antigen-antibody complexes activated C1r and C1s
>>C1 inhibitor (C1INH) dissociates C1r and C1s from active C1 complex 2. C4b2a is the active C3 convertase, cleaving C3 to C3a and C3b >> DAF, C4BP and CR1 displace C2a from C4b2a complex. C4b bound by C4BP, MCP or CR1 is cleaved by a soluble protease I to inactive forms C4d and C4c 3. C5 convertases cleave C5 to C5a and C5b >> CR1 and H displace C3b. CR1 and H act as cofactors in cleavage of C3b by I 4. Terminal components of complement form a membrane pore:MAC >>Cd59 prevents final assembly of MAC at C8 to C9 stage |
|
|
Generation of C5 convertase
|
1. C3b binds to both C4b2a and C3bBb, forming active C5 convertases C4b2a3b and C3b2Bb
2. C5 binds to C3b component of the C5 convertase enzyme 3. C5 is cleaved by C2a or Bb to form C5b or C5a |
|
|
anaphylotoxin C5a enhances phagocytosis
|
1. bacterium is coated with complement by the alternative and MBL pathways
2. When only C3b binds to CR1, bacteria are not phagocytosed 3. C5 can activate macrophages to phagocytose via CR1 |
|
|
what are the other anaphylotoxins?
|
*small components of C3a, C4a and C5a when produced in large amounts or injected systemically, can induce generalized circulatory collapse (like a systemic allergic reaction involving antibody of the IgE class)
|
|
|
what are local inflammation induced by complement fragments?
|
1. small complement-cleavage products (C3a, C5a and C4a) act on BVs to increase vascular permeability and cell-adhesion molecules
2. increased permeability allows increase fluid leakage from BVs and let out Ig (IgG and IgM) and complement molecules 3. migration of macrophages, PMNs and lymphocytes is increased. Microbicidal activity of macrophages and PMNs is also increased. |
|
|
Stages where complement activity id regulated
|
1. C1q binding to antigen-antibody complexes activated C1r and C1s
>>C1 inhibitor (C1INH) dissociates C1r and C1s from active C1 complex 2. C4b2a is the active C3 convertase, cleaving C3 to C3a and C3b >> DAF, C4BP and CR1 displace C2a from C4b2a complex. C4b bound by C4BP, MCP or CR1 is cleaved by a soluble protease I to inactive forms C4d and C4c 3. C5 convertases cleave C5 to C5a and C5b >> CR1 and H displace C3b. CR1 and H act as cofactors in cleavage of C3b by I 4. Terminal components of complement form a membrane pore:MAC >>Cd59 prevents final assembly of MAC at C8 to C9 stage |
|
|
what are the 3 families of adhesion molecules important for leukocyte recruitment?
|
1. Selectins
- membrane glycoproteins with distal lectin-like domain that binds specifically to carbo group - induced on activated endothelium and initiate endothelium-leukocyte interactions by binding to fucosylated oligosaccharide ligands om passing leukocytes 2. Integrins: - bind to cell-adhesion molecules and Extra Cellular Matrix (ECM), strong adesion -ie: ICAMs (Inter Cellular Adhesion Molecules) 3. Immunoglobulin Superfamily -various roles in cell adhesion -ligands for integrins -not in antibody but has the same shape as Ig (antibody) |
|
|
Phagocyte adhesion to vascular endothelium(in BV linings) is mediated by integrins
|
- when vascular endothelium is activated by inflammatory mediators it expresses 2 adhesion molecules:
* ICAM-1 * ICAM-2 they are ligands for integrins, expressed by phagocytes |
|
|
Multi-steps process involving adhesive interactions regulated by macrophage-derived cytokines and chemokines
|
Selectin-mediated adhesion to leukocyte sialyl-Lewis (s-LeX) is weak and allows leukocytes to roll along vascular endothelial surface
* rolling adhesion * tight binding: selectin gets the cell to slow down * diapedesis * migration |

