![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
9 Cards in this Set
- Front
- Back
|
Describe the structure of antibodies.
|
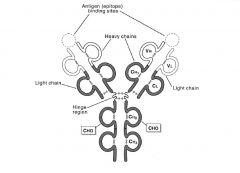
-it is composed of 2 heavy chains and 2 light chains, connected by disulfide bridges
-the antigen binds to the N-terminus of both chains -each light chain has a variable domain which binds the antigen & a constant domain which does not -there are 2 light chain constant domains in the genome: the kappa and lambda domains -the heavy chain has 1 variable domain which binds the antigen and 3 constant domains which do not bind the antigen -different constant parts of the antibodies give rise to antibodies with different isotypes ie. IgM, IgD, IgE, IgG |
|
|
What is an epitope, what is an idiotope?
|
Epitope - is the part of the antigen that is recognized by the adaptive immune system
-there are B cell epitopes (ie. parts of antigens that are recognized by B cells and antibodies) and T cell epitopes (ie. parts of the antigen recognized by T cells through their T cell receptor) Idiotope - is the part of the antibody that binds the antigen -defined by the combination of the variable region of the heavy and light chains of the antibody |
|
|
What is affinity vs avidity?
|
“Affinity” is the strength of binding of one molecule to a ligand. For example, the strength of binding of an antibody to an antigen.
However “avidity” is different from “affinity” as avidity is the sum of total strength of binding of more than one molecules to ligands. The difference between them is that affinity is the binding strength of one molecule site to its ligand, but avidity and affinity are correlated. The strength of avidity depends on the affinity and the valency of interactions. For example, immunoglobulin M (IgM) (one type of antibodies, see figure on the right) has a pentameric shape and 10 antigen-binding sites. The multivalent IgM is able to bind to more antigens than other antibodies, such as IgG. To simply things, affinity is just like how close you can be with a single person. Avidity represents how many friends you can have. |
|
|
Describe the complementarity of antibodies and antigen
|
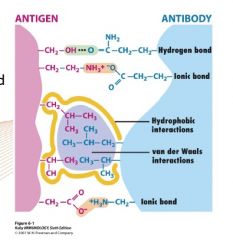
Complementarity is not only topological (ie. shape, short range van der Waals interactions play a role) but also in terms of hydrogen bonds and electrical charge of the amino acids interacting
|
|
|
Describe the characteristics of B cell epitopes
|
♦Always exposed on the surface
♦3D structures are recognized -therefore vaccines relying on B cell humoral immunity need to have correctly folding antigens -carbohydrates can be important in B cell epitopes |
|
|
Describe the characteristics of T cell epitopes
|
♦T cells generally recognize a complex between a "self" molecule and a "foreign" peptide
♦T cells recognize only small peptides that are presented to the T cell receptor in association with the major histocompatibility MHC antigen on the surface of cells ♦The MHC molecule on the surface of the antigen presenting cells resembles a BBQ with 2 self “sausages” sitting on the top. The T cell epitope peptide sits between the two sausages on top of the grill. The grill is a β-pleated sheet while each of the self “sausages” is an α-helix. The T cell epitope peptide can have different conformations but needs to fit in the grove between the two sausages on top of the grill. ♦MHC molecules are variable from one individual to the other (except identical twins or cloned animals). This variation accounts for some of the differences between individuals in susceptibility to diseases ♦The process of transforming antigen to small peptides presented to T cells in association with MHC is called antigen processing and the process of presenting this processed antigen to T cells is called antigen presentation. As a result of antigen processing the peptides presented to the T cells: ✤Can be located anywhere in the protein (i.e. surface exposed or hidden within the antigen). The antigen themselves can be either exposed on the surface of the pathogen or internally. ✤Have to be in a linear sequence (i.e. a continuous stretch of amino acid in the protein sequence). ✤Carbohydrates can affect how the protein is processed and presented. ✤There are non-protein T cell epitopes but they are the exception rather than the rule |
|
|
What class of cells express MHC I, MHC II? What T cell recognizes these receptors?
|
✤MHC class I (MHC I) interacts with CD 8 T cells and recognise T cell epitopes of about 8-10 amino acids. MHC I is expressed on most nucleated cells
✤MHC class II (MHC II) interacts with CD4 T cells and recognise T cell epitopes of less well defined lengths (13-25 amino acids). MHC II is expressed on antigen presenting cells (APC) |
|
|
What is the function of T helper cells? Cytotoxic T cells?
|
T-helper cells are also called CD 4+ T cells and their function is to help other cells mount immune responses. They regulate the immune response and help decide which type of immune response is induced. They express the CD 4+ molecule which interacts with the constant part of the MHC II molecule
Cytotoxic T cells (CTL) are also called CD 8+ T cells and their primary function is to lyse infected target cells. They express the CD8+ molecule which interacts with the constant part of the MHC I molecule |
|
|
What does an NK T cell recognize & what kind of receptor does it have?
|
A subset of lymphocytes called NK T cells recognises an MHC-like molecule (CD1d) in association with glycolipids such as α-Galactosylceramide.
|

