![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
50 Cards in this Set
- Front
- Back
|
What chemical mediators in acute inflammation are responsible for the cardinal signs of inflammation?
|
Rubor/calor: histamine-mediated vasodilation of arterioles
Tumor: histamine-mediated increase in permeability of venules Dolor: PGE2 sensitizes nerve endings to the effects of bradykinin |
|
|
Describe the sequential vascular events that occur in an acute inflammation that enable leukocytes to reach an infection.
|
1) Neurogenic reflex (vasoconstriction). 2) Vasodilation (histamine, NO). 3) Increased permeability of venules (histamine, IL-1, TNF). 4) Edema (outflow of fluid > lymphatic drainage
|
|
|
Describe the sequential cellular events that occur in an acute inflammation that enable leukocytes to reach an infection.
|
1) Margination (neutrophils move to the periphery). 2) Rolling (selectin adhesion molecules). 3) Adhesion (B-integrins on neutrophils, CAMs on ECs). 4) Diapedesis (neutrophils dissolve BM with IV collagenase, edema carries opsonins). 5) Chemotaxis (C5a, LTB4, IL-8)
|
|
|
Which molecules activate neutrophil adhesion molecules in actue inflammation?
|
C5a and LTB4 activate B2-integrin on neutrophils. B2-integrin binds to ICAM and VCAM on endothelial cells.
|
|
|
What is the most common exogenous agent on bacteria that acts as a chemoattractant?
|
N-formyl-methionine terminal amino acid
|
|
|
What are the three main steps of phagocytosis?
|
Opsonization (IgG, C3b, C-reactive protein), ingestion (phagolysosome formation), killing.
|
|
|
Which leukocytes can produce bleach? Describe the oxidative burst.
|
Neutrophils and monocytes (not macrophages). NADPH oxidase oxidizes NADPH to NADP while reducing molecular oxygen to form superoxide free radicals. These free radicals are then converted to peroxide via superoxide dismutase. MPO combines peroxide with Cl ions to form bleach.
|
|
|
What molecule neutralizes hydrogen peroxide?
|
Glutathione peroxidase.
|
|
|
Name the oxygen-independent compounds used for bacterial killing.
|
Acids, lysosomes (degrades bacterial peptidoglycan: acid-N-acetyl-glucosamine), lactoferrin (iron chelator), defensins (cytoxoic to microbes), major basic protein (from eosinophils and cytotoxic to parasites)
|
|
|
What is arachodonic acid metabolism?
|
In response to several stimuli, cells use membrane lipids to generate biologically active lipid mediators (autocoids, short acting hormones) that aid in inflammation and hemostasis.
|
|
|
What essential acid is arachadonic acid made from? What are arachadonic metabolites also known as?
|
Linoleic acid. Metabolites are eicosanoids.
|
|
|
What are the arachadonic acid metabolites and what enzymes form them?
|
Phospholipase A2 converts membrane phospholipids to arachadonic acid.
COX converts AA to PGG2, PGH2, PGE2. PGH2 is converted to PGI2 (prostacyclin synthase) and TXA2 (thromboxane synthase). Lipoxygenase converts AA to LTB4, LTC4, LTD4, LTE4. |
|
|
What are the functions PGE2? PGI2? LTB4? LTC,D,E?
|
PGE2: VD, pain, fever, keeps ductus areteriosus open)
PGI2: VD, inhibit platelet aggregation LTB4: chemotaxis, activates neutrophil adhesion molecules LTC,D,E: VC, increase vessel permeability, bronchoconstriction |
|
|
Complement pathways differ initially, but both form C3 and C5 convertase and ultimately MAC. What are the complement pathways?
|
Alternate pathway: activated by microbial surfaces (LPS, teichoic acid)
Classical pathway: activated by Ag-Ab Mannose-lectin pathway |
|
|
What are the important cleavage products in the complement system?
|
C3b and C4b – opsonize
C3a and C5a – chemotaxis (neutrophils, monocytes) C3a and C5a (anaphylotoxins) – release of mediators from mast cells/basophils (histamine, serotonin, PG's) |
|
|
Name the types of acute inflammation.
|
Purulent: pus-forming organisms
Fibrinous inflammation: deposition of a fibrin exudate Pseudomembranous inflammation: bacterial toxin-induced damage of mucosal lining (C. difficile, C. diphtheriae) |
|
|
What is the significance of a fever in inflammation?
|
Right-shifts O2-binding curve. More O2 available for oxidative burst.
|
|
|
What is granulation tissue?
|
Highly vascular tissue composed of new blood vessels (angiogenesis) and activated fibroblasts. Essential for normal wound healing. Converted to scar tissue.
|
|
|
What is the key adhesion glycoprotein in ECM required for granulation tissue?
|
Fibronectin.
|
|
|
A special type of chronic inflammation is known as granulomatous inflammation caused by infections or noninfections (sarcoidosis, Crohn's). What is the morphology of a granuloma?
|
Pale, white nodule with/without caseous necrosis. Epithelioid cells (activated macrophages) surrounded by CD4 helper T cells, Th1. Multinucleated giant cells are formed by the fusion of the epithelioid cells.
|
|
|
In tuberculous granuloma, macrophages present Ag to CD4 T cells and then secrete what in order to stimulate Th1 formation/activation. Subsequently, Th1 cells release what factors to control macrophage activity?
|
Macrophages secrete IL-12 for Th1 formation and IL-1 for fever.
Th1 cells release IL-2 (stimulate Th1 proliferation), gamma-interferon (activate macrophages), MIF (migration inhibitory factor which causes macrophage accumulation). |
|
|
In fibrosis, what collagen is initial produced? Metalloproteinases replace type III collagen with _____ collagen.
|
Type III collagen. Metalloproteinases replace type III collagen with type I collagen.
|
|
|
Of the three types of cells, which can undergo cell regeneration? Labile, stable, permanent.
|
Labile (stem cells) and stable (e.g. fibroblasts) can replicate. Permanent (cardiac and striated muscle) cannont.
|
|
|
What cells in the lung are responsible for repair?
|
Type II pneumocytes which replace both type I and type II.
|
|
|
What is gliosis?
|
Astrocyte proliferation in the brain in response to an injury (brain infarction).
|
|
|
What are some causes of neutrophil leukocytosis?
|
Infection, sterile inflammation with necrosis (acute MI), drugs inhibiting neutrophil adhesion molecules: corticosteroids, catecholamines, lithium.
|
|
|
What is a 'left shift' of leukocytes referring to?
|
Defined as greater than 10% band (stab) neutrophils or the presence of earlier precursors.
|
|
|
What are azurophilic granules?
|
Primary lysosomes (contains MPO, lysosomes, defensins, etc)
|
|
|
Patient presents with recurrent bouts of swelling in his face and oropharynx. What is the diagnosis and pathophysiology?
|
Hereditary angioedema. Autosomal disorder with deficiency of C1 esterase inhibitor which leads to increase in anaphylatoic activity.
|
|
|
What is the most common complement deficiency?
|
C2 deficiency. Associated with septicemia and lupus-like syndrome in children.
|
|
|
Child presents with disseminated Neisseria gonorrhoeae. Last month he was infected with N. menignitidis. What disorder could he have?
|
C6-C9 deficiency. No MAC complex which normally kills unencapsulated gram negative bugs.
|
|
|
Patient presents with hemoglubinuria and anemia. You determine his condition is related to a missing membrane protein. What is it?
|
Decay accelerating factor (DAF) normally degrades C3 and C5 convertase on hematopoietic cell membranes. The patient has paroxysmal nocturnal hemoglobinuria.
|
|
|
If there is a decrease in factor B, what complement pathway is activated?
A decrease in C4 or C2 indicates activation of which pathway? |
Alternative pathway
Classical pathway |
|
|
A child presents with severe gingivitis, poor wound healing, peripheral blood and neutrophilic leukocytosis. Additionally, his mother says that his umbilical cord took an abnormally long time to separate. What is the diagnosis?
|
Leukocyte adhesion deficiency (LAD). An autosomal recessive disorder. LAD1 is deficient in CD11a:CD18 (integrin). LAD2 is deficient of a selectin that binds to the neutrophils.
|
|
|
Patient presents with recurrent infections, photophobia and pale skin. A staining of his leukoctyes show giant azurophilic granules. What is the diagnosis?
|
Autosomal recessive Chediak-Higashi syndrome which is a defect in membrane fusion. Characterized by defects in chemotaxis, degranulation, and bacteriocidal activity (cant form secondary phagolysosomes).
|
|
|
A patient who suffered from psychomotor retardation died at age 9. His lysosomes contained no hydrolytic enzymes. What is the pathophysiology of his disease?
|
I cell disease: Defect in phosphorylation of mannose in glycoproteins that target proteins to lysosomes. Inclusion bodies accumulate in cytosol.
|
|
|
What are some clinical features of I cell disease?
|
Skeletal abnormalities, coarse features, restricted joint movement, psychomotor retardation.
|
|
|
What is the adult form of I cell disease?
|
pseudo-Hurler polydystrophy.
|
|
|
What type of mutation causes Tay-Sach's disease?
|
Frameshit: four base insert results in the synthesis of a defective lysosomal enzyme (hexosaminidase).
|
|
Patient also present with dysarthria, dysphagia, ataxia, and spasticity.
|
Tay-Sachs disease. GM2 ganglioside build up to toxic levels within nerve cells of the brain.
|
|
|
What two disease result in a build up of mucopolysaccharides (dermatan and heparan sulfate)?
|
Hurler's (autosomal recessive) and Hunter's (X-linked). L-iduronidase deficiency. Characterized by multiple organ involvement, coarse facial features, retardation, corneal clouding, and coronary artery disease
|
|
|
What lysosomal storage disease is deficient in glucocerebrosidase? What characteristic cells are present?
|
Gaucher's disease (adult type I) is characterized by fibrillar appearing macrophages.
|
|
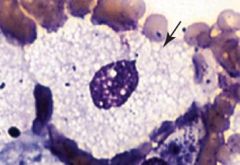
Notice the soap-bubble appearance of the macrophage. What is accumulating?
|
Sphingomyelinase. Niemann-Pick disease .
|
|
|
Red headed patient presents with severe eczema and very elevated IgE.
|
Job's syndrome aka hyper-IgE syndrome.
|
|
|
Boy presents with Staphylococcus and Burkholderia infections. WBC profile and serum antibody levels in the high-normal range. Neutrophils failed to reduce NBT. Diagnosis?
|
The boy has chronic granulomatous disease.
|
|
|
What is the genetic basis of chronic granulomatous diease?
|
65% are X-linked and 35% are autosomal recessive.
|
|
|
How come CGD often presents with recurrent staph infections but no streptococcal infections?
|
Streptococcus are catalase negative. The bacteria provides hydrogen peroxide to the phagocyte which then produces HOCl (hypochlorite)
|
|
|
What are the common infections in chronic granulomatous disease?
|
Aspergillus, Candida, Staph aureus, Serratia, Burkholderia cepacia.
|
|
|
What cells release IFN-gamma?
What are some functions of IFN-gamma? What phagocytic disorder can it be used to treat? |
Th1, NK cells. IFN-gamma enhances the activity of macrophages, inhibits Th2 response, and upregulate expression of MHC molecules. Its used to treat CGD, because it increases macrophage ability to kill bacteria.
|
|
|
What infections are patients with IFN-gamma receptor deficiency susceptible to?
|
Defective IFN-gamma receptor prevents macrophage activation and killing of intracellular pathogens. These patients can have disseminated, severe mycobacterial infections.
|

