![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
227 Cards in this Set
- Front
- Back
|
What is the advantage of employing apoptotic death?
|
Death is mediated by apoptosis. It is a controlled way for cell death, confining the destruction to a pinpointed area. Stop viral replication, preventing release of infective viral components into system. It also prevents unctonrolled release of necortic debris which could trigger inflammaotry response.
|
|
|
How does cytotoxicity occur?
|
1. Interaction of CTL and target cells. Granules are dispersed in the T cell.
2. Immunological synpase – all the granules migrate. Decision to attack or not attack is made. 3. Polarization (all the granules are found adjacent to target cell) 4. Polarized secretion of cytotoxic granules 5. Death of target cell, T cell dissociates and is ready for another cycle of killing |
|
|
What is the immunological synpase? How does it function?
|
Supramolcular adhesion complex (SMAC) enables clustering of signals, focused secretion of cytotoxic granules, confinement of effect.
1. Initial interaction: LFA1/ICAM, CD2/CD58 2. Antigen-specific recognition causes conformational change of LFA-1 = release of effector molecules 3. Cell separate 4. Target cell dies |
|
|
Where is the primary lymph drainage in each of the following sites?
1. upper limb, lateral breast 2. stomach 3. duodenum, jejunum 4. sigmoid colon 5. rectum (LOWER PART) 6. anal canal (ABOVE PECTINATE LINE) 7. testes 8. scrotum 9. thigh (superifcial) 10. lateral side of dorsum of foot |
1. upper limb, lateral breast
AXILLARY 2. stomach CELIAC 3. duodenum, jejunum SUPERIOR MESENTERIC 4. sigmoid colon COLIC --> INFERIOR MESENTERIC 5. rectum INTERNAL ILIAC 6. anal canal SUPERFICIAL INGUINAL 7. testes SUPERFICIAL AND DEEP PLEXUS --> PARA-AORTIC 8. scrotum SUPERFICIAL INGUINAL 9. thigh (superifcial) SUPERFICIAL INGUINAL 10. lateral side of dorsum of foot POPLITEAL |
|
|
A3
|
hemochromatosis
|
|
|
What triggers the release of cytotoxic granules from the T cell?
|
Calcium
|
|
|
What are the three cytotoxic granules?
|
1. Perforin – polymerized, generate holes in attack cells
2. Granzymes – proteases that penetrate into the attack cell and start cleaving various elements to trigger apoptosis 3. Serglycin – scaffold that holds perforin and granzyme |
|
|
Describe the steps taken to induce apoptosis by cytotoxic granules.
|
1. T cell decides to attack, it releases serglycin, which holds the perforin and granzymes.
2. Perforin creates hole, granzymes goes in and targets BID and pro-capase 3. The cleaving of BID disrupts MCOM and activated capase-3 cleaves ICA, releaseing capase activated DNase (CAS) 4. Release of cytochrome c into cytosol activates apoptoses, and CAD induces DNA fragmentation |
|
|
What are granule-independent cytotoxic activity? Describe them in detail.
|
Fas-Fas ligrand interactions. They belong to the TNF family, part of type I TNF receptors. Direct cell-cell interaction. Fas=FasL interactions causes activation of death signals by Fas.
|
|
|
What are the physiologic roles of Fas-Fas ligand interactions?
|
1. Termination of the immune response
2. Part of barrier in immume privileged sites |
|
|
What is the only known way in which CD4 can directly kill a cell?
|
Through FasL
|
|
|
How can FasL be utilized to the benefit of tumor cells?
|
By triggering death signals in T lymphocytes.
|
|
|
What is the purpose of activating receptors? What do they recruit? What are some examples?
|
1. In charge of identification of “danger” signals – MHC: peptide, PAMPs, stress markers
2. Transduce activating signals mostly via ITAM 3. Recruit kinases Examples: TCR, natural cytotoxcitiy receptors, NKG2D |
|
|
What is the purpose of inhibiting receptors? What do they recruit? What are some examples?
|
1. Bind various ligands (self proteins, non-self proteins)
2. Transduce inhibitory signals via ITIM 3. Recruit phosphatases Examples: Killer Ig-like inhibitory receptors (KIR), leukocyte receptors (LIR) |
|
|
What are the functions of CD8+ T cells?
|
Killing activity
Cytokine secretion Proliferation |
|
|
Which cells have MHC I expression? What effect does enhanced expression have?
|
Expressed by all nucleated cells. All cells are susceptible to viral infection, mutation, and tumor formation.
Enhanced expression leads to enhanced recognition by CD8+ T cells. |
|
|
Describe signaling by TCR activation (10 steps).
|
1. In the resting T cell, the ITAMs are not phosphorylated
2. Binding of ligand to the receptor leads to phosphoyrl. of the ITAMs by Lckw when the co-receptor binds to the MHC ligand 3. ZAP-70 binds to the phosphorylated chain of ITAMs and is phosphorylated and actived 4. Activated ZAP-70 phosphoryl. LAT and SLP-76 5. GADS brings SLP-76 and LAT together 6. GADS:SLP-76:LAT complex receruits PLC-gamma 7. PLC-gamma is activated by phosphoryl. by Itk 8. PLC cleaves PIP2 into DAG and IP3 8. IP3 opens Ca channels to allow Ca entry from ER. Depletion of Ca from ER leads to opening of CRAC channels in the plasma membrane allowing entry of extracellular Ca. 9. IP3 opens Ca channels to allow Ca entry from the ER. Depletion of Ca from the ER leads to opening of CRAC channels in the plasma membrane allowing entry of extracellular Ca 10. DAG remains in the membrane and recruites PKC-8 and RasGRP to membrane |
|
|
How are effector CTLs generated?
|
1. Naive CTL, then with appropriate Ag presentation
2. Signal 1 + 2, then with help from Th1 cells 3. Signal 3 (IL-2, IFN-gamma) 4. Differentiate into effector CTL and memory CTL, then with appropriate Ag presentation of memory CTL 5. Signal 1+2+3 (endogenous high IL-2 production) 6. Differentiate into effector CTL |
|
|
How do CD8 cells recognize tumors?
|
Once an APC picks up tumor antigens from the environment, most of it goes to exogenous presentation and pathway, but some elements become translocated from endosome into cytoplasm. (Unknown mech). Once they become cytosolic, the antigens enter the endogenous pathway.
|
|
|
How can an NK cell be IDed? What are its effector functions?
|
By lack of CD and presence of CD56
1. cytotoxic activity 2. cytokine secretion activity (IFNg, TNFa) |
|
|
What secretes IL-2? What does it act on? What are its action?
|
1. Th1 cells
2. T cells, B cells, NK cells 3. T cell Proliferation (CTL proliferation and growth) B cell Activation NK Cell Activation |
|
|
How do NK cells decide whether or not to kill a cell? Where does this happen? What is this process based on?
|
Differentiation b/w self and non-self is done through the immunological synapse and is tightly regulated through use of inhibitory and activating signals.
Based on recognition of MHC class I = "self-sign." MHC I are altered in virus-infected and cancer cells. |
|
|
What two families of inhibitbory NK receptors exist
|
1. Ig superfamily, mostly HLA-C, -B
2. C-type lectins, many HLA-E |
|
|
What are some ways viruses manipulate NK inhibitory functions to its benefit? Focus on CMV, HIV, Flu
|
1. CMV -- UL18 protein is MHC class I-like homologue; inhibits NK cells
2. CMV -- UL40 contains pepride that binds to HLA-E 3. HIV -- Nef protein selectively downregulateds HLA-A and -B, but not -C 4. Flu -- works on all MHC receptors, clusterization of MHC class I to increase binding avidity 5. CMV -- induction of other inhibitory ligands |
|
|
How are RBCs protected from NK cells?
|
The NK cell must get a positive activating signal. RBCs do not express MHC I, so no negative signal, but they do not express any danger signals either. There are not recognized either which way.
|
|
|
What are the humoral responses? What happens in each Which antibodies predominate?
|
1. Primary Response -- Naive cells, IgM (higher affinity b/c forms the pentamer)
2. Secondary Response -- memory cells, IgG |
|
|
Where do B cells mature before birth? after birth?
|
1. In the yolk sac, fetal liver, fetal bone marrow
2. Bone marrow |
|
|
What are the phases of cell maturation?
|
1. Generation of B cells in bone marrow
2. Elimination of self-reactive B cells in bone marrow 3. Activation of B cells by foreign antigen in secondary lymphoid tissues 4. Differentiation to antibody secreting plsma cells and memory B cells in secondary lymphoid tissues |
|
|
Describe the early stage of generation of B cells in bone marrow.
|
1. Stem progenitor cells attach to stroma via VLA-4 and VCAM-1
2. Formation of early progenitor cell, which expresses Kit and attaches to the SCF receptor on the stroma 3. Signals B cell to express IL7 receptor and the stroma to generate IL7 cytokinae --> pre-B cell |
|
|
How is the development of a B cell marked by rearrangement?
|
1. early pro B: heavy chain DJ rearrangement
2. late pro-B: heavy chain VDJ rearrangment 3. small pro B: light chain VJ rearrangement |
|
|
Describe the process of activation of B cell by a foreign antigen.
|
1. Thymus independent -- 2 signals in the same cell cause cross linking of FAB by antigen and signal transduction independent of T cell interference
2. Thymus dependent -- requires T helper cells as the 2nd messenger, occurs in germinal cells and paracortex |
|
|
How does B cell activation to occur?
|
ITAM sits on alpha and beta sububits and interacts with several members of src family of tyrosine kinases. Activated enzymes phosphorylate tyrosine residues on the cytoplasmic tails of the heterodimer, creating docking site for Syk kinase, which is then also activated.
|
|
|
What can enhance B cell response? Describe this complex. What does it contain. What does it do?
|
B cell coreceptor complex.
Has CD-21, 19, 81. Can bind an antigen, which will also bind to the Fab of the B cell receptor, causing cross-linking. Can induce ITAM. Does not undergo a class switch, mostly IgM. No immunological membrane. |
|
|
What are ten warning signs of an immunodeficient disease?
|
1. Eight or more infection within a year
2. 2 or more serious sinus infections w/i a year 3. 2 or more monthd on antibodies with little effect 4. Two or more pneumonias a year 5. Failure of an infant to gain weight or grow normally 6. Recurrent, deep skin or organ abscesses 7. Persistent thrush in mouth or elsewhere on skin 8. Need for intravenous antibiotics to clear infections 9. Two or more deep-seated infections 10. A family history of primary immunodeficiency |
|
|
What is the difference b/w primary and secondary immunodeficiencies? What are some examples?
|
Secondary -- immunodef. is not the main problem. There is another disease or organ that is infected.
Chronic diseases, HIV, Nephrotic Syndrome, Medications, GERD with aspiration |
|
|
What are the causes of primary immunodefs (list in order of most common to least)?
|
1. Antibody deficiencies (655%)
2. Combined cellular and antibody def (15%) 3. Phagocytic defic (10%) 4. Cellular defic (5%) 5. Complement defic (5%) |
|
|
Name some common Antibody Deficient Primary Immunodeficiencies.
|
X-Linked Agammaglobulinemia (Bruton’s disease)
Common Variable Immune deficiency Transient Hypogammaglobulinemia of infancy Hyper IgM syndrome (CD 40 ligand deficiency) IgA deficiency |
|
|
Name some common Cell-Mediated Primary Immunodeficiencies.
|
DiGeorge Syndrome
Chronic Mucocutaneous Candidiasis |
|
|
Name some common Complement Primary Immunodeficiencies.
|
C2 Deficiency
C5-9 Deficiency |
|
|
Name some common Combined Primary Immunodeficiencies.
|
Severe Combined Immunodeficiency (SCID)
Wiskott-Aldrich Syndrome Ataxia Telangiectasia X-Linked Lymphoproliferative Disease |
|
|
Name some common Phagocytic Defects Primary Immunodeficiencies.
|
Phagocytic Immunodeficiencies
Chronic Granulomatous Disease Leukocyte Adhesion Deficiency |
|
|
How can primary immunodef be diagnosed? (generally)
|
CBC, diff, smear
Electrolytes protein, albumin blood culture, sputum culture, stool cultures, HIV as indicated sweat chloride chest x-ray, sinus x-ray |
|
|
Describe immunodef. What defects exist?
|
Defect in B-cell production and/or function
Clinical symptoms related to lack of immunoglobulin production both specific and non specific Some effect on T-cells secondary to T and B cell interaction |
|
|
How is humoral immunity evaluated?
|
1. Quantitative immunoglobulins: IgG, IgA, IgM, IgE
2. (IgG subclasses) 3. Levels of specific antibodies to polio, diptheria, tetanus, measles, mumps, rubella 4. Response to pneumococcal vaccine 5. Isohemagglutinin ( 6. Lymphocyte subsets (to evaluate B cell numbers) |
|
|
Describe X-linked Agammaglobulinemia. Who is infected? What are symptoms? What are clinical complications?
|
Boys 4-12 months
1. Recurrent bacterial infections 2. Pyogenic bacteria S. pneumoniae, H. influenzae and strep 3. Chronic infections 4. Septic and non-infectious arthritis 5. Meningitis 6. Enteroviral infection 7. PCP infection 8. XLA and cancer |
|
|
Describe XLA pathophysiology? What is lacking? What is seen? Where is the genetic defect?
|
1. Major abnormality is lack of B lymphocyctes in peripheral blood and other organs (<2% CD19+)
2. Absence of immunoglobulin production 3. Rudimentary lymph tissue with abnormal histology 4. Absent plasma cells 5. Bone marrow shows arrest of B cell maturation 90 - 95% of patients with XLA have a BTK mutation |
|
|
What is common variable immunodeficiency? How can it be described? What is the mechanism? Clinical presentation? Onset?
|
most common primary immunodeficiency
“mixed bag” of disorders hypogammaglobulinemia and impaired antibody responses variable T-cell function age at onset of disease early childhood and 18 to 25 years recurrent sinopulmonary infections |
|
|
What are disease states related to CVI?
|
Deteriorating pulmonary status resulting in permanent irreversible lung damage
GI disorders mycoplasma infection enteroviral infection Autoimmune disease granulomatous inflammation |
|
|
What is cell-mediated immunodef? What genetic mutations is it related to? What developmental abnormalities?
|
Defects in T-cell production and/or function
affects acquired immunity leading to risks of opportunistic infections related to genetic mutations affecting: receptors signaling enzyme deficiencies related to developmental abnormalities: thymic maturation |
|
|
How is cell mediated immunity evaluated?
|
Measurement of T and B-cell subsets
intradermal skin testing with heat-killed Candida in vitro lymphocyte proliferation in response to mitogens, antigens and allogenic cells-- (specialized testing) Adenosine deaminase and purine nucleoside phosphorylase levels-- (specialized testing) thymic biopsy-- (specialized testing) |
|
|
What are the four main mechanisms that lead to a deregulation of T cell development?
|
1. Premature cell death due to toxicity (T-B-NK-)
2. Defective cytokine-dependent survival signaling (T-B+NK-) 3. Defective V(D)J rearrangement of T cell receptor (T-B-NK+) 4. Defective pre-TCR and TCR signaling (T-B+NK+) |
|
|
What are clinical presentations of SCID?
|
Age - infancy
Failure to thrive Recurrent viral, fungal, protozoal and bacterial infections Paucity of lymphoid tissue in most types Skin and skeletal abnormalities Haematological abnormalities Previous family history of early deaths |
|
|
How can SCID be diagnosed and evaluated?
|
Total lymphocyte numbers
Humoral immunity T & B cell numbers (subsets) In-vitro lymphocyte function Candida skin test Skin graft rejection |
|
|
What is lymphocyte immunotyping? How is this used in diagnosing SCID? What are some pertinent results?
|
LOOK THIS UP
CD19 + (Pan B cells) low CD3-/CD(16+56) + (NK Cells) high Average CD3 + (Mature T) low RAG1 deficient SCID (T-B-NK+ SCID) CD19 + (Pan B cells) high CD3-/CD(16+56) + (NK Cells) high Average CD3 + (Mature T) low CD3d deficient SCID (T-B+NK+ SCID) |
|
|
What is a thymidine incorporation proliferation assay? What is it measuing? How is it used? What does it diagnose?
|
LOOK THIS UP
|
|
|
Go over variable beta repertoire.
|
LOOK THIS UP
|
|
|
How is passive immunity acquired?
|
1. Natural maternal antibody
2. Immune globulin 3. Humaized monoclonal antibody 4. Antitoxin |
|
|
How is active immunity acquired?
|
1. Natural infection
2. Vaccines (attenuated, inactivated, purified microbial, cloned microbial -- recombinant of cloned DNA, multivalent complexes) |
|
|
What are the roles of IL-15 and IL-7?
|
Cytokines can affect T-cell proliferation and survival at many stages of the immune response. During initiation of the T-cell response, interleukin-15 (IL-15) might be involved in dendritic-cell (DC) activation. After T-cell receptor (TCR) ligation of peptide–MHC, substantial T-cell clonal expansion occurs and might be driven, in part, by IL-2. IL-15 might also enhance the proliferation of antigen-specific T cells. IL-2 can also control the late clonal-expansion phase by inducing T-cell death. The massive cell death that occurs during the contraction phase results in the loss of most antigen-specific T cells. Both IL-15 and IL-7 might rescue T cells from cell death at this stage, thereby allowing memory T-cell generation. Memory T cells are maintained long term by undergoing a low level of proliferation, which depends on IL-15. IL-7 seems to be more important for promoting the survival, rather than the growth, of memory T cells.
|
|
|
How are CD 8 memory cells generated and differentiated?
|
After antigen presentation, CD8+ naive T cells are activated and differentiate into memory stem cells, central memory and effector memory CD8+ T cells. The differentiation pathways are known (solid) or unproven (dotted). Memory stem cells self-replicate and differentiate into both central memory and effector memory, although the latter may be indirect. Central memory CD8+ T cells have a limited ability to generate memory stem cells. Effector memory CD8+ T cells are terminally differentiated and cannot generate other cell types. Like naive CD8+ T cells, memory stem cells express low levels of CD44 and high levels of CD62L, but they are uniquely Sca-1 positive.
|
|
|
Describe generation and differentation of memory T cells.
|
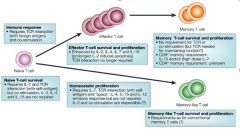
|
|
|
What are common agents used for passive immunization?
|
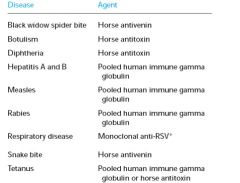
|
|
|
What are different types of active vaccine?
|
Live, attenuated vaccines
Inactivated vaccines Subunit vaccines Toxoid vaccines Conjugate vaccines DNA vaccines Recombinant vector vaccines |
|
|
What are the features of an effective vaccine?
|
1. Safe
2. Protective 3. Sustained protection 4. Induces neutralizing antibody 5. Induces protective T cells 6. Practicality (cost, ease of administration, side effects) |
|
|
How are attenuated viruses selected?
|
1. Isolated from patient and grown in human cultured cells
2. Cultured virus is used to infect monkey cells 3. Virus acquires many mutations that allow it to grow well in monkey cells 4. Virus no longer grows well in human cells (it is attenuated) and can be used as vaccine |
|
|
Why are recombinant DNA techniques especially useful?
|
Attenuation can be achieved more rapidly and reliably with recombinant DNA techniques
1. Isolate pathogenic virus 2. Isolate virulence gene 3. Mutate of delete the gene 4. Resulting virus is viable, immunogenic, but avirulent. Can be used as vaccine |
|
|
What are toxoid vaccines? How are these used? What is the mechanism? Are they safe? What are they used against?
|
For bacteria that secrete toxins, or harmful chemicals, a toxoid vaccine might be the answer. These vaccines are used when a bacterial toxin is the main cause of illness.
Toxins can be inactivate by treating them with formalin, a solution of formaldehyde and sterilized water. Such “detoxified” toxins, called toxoids, are safe for use in vaccines. The immune system produces antibodies that block the toxin. Vaccines against diphtheria and tetanus are examples of toxoid vaccines. |
|
|
What are conjugate vaccines? How are they made? What do they protect against? Why are they important?
|
Many harmful bacteria possess an outer coating of sugar molecules called polysaccharides. Polysaccharide coatings disguise a bacterium’s antigens so that the immature immune systems of infants and younger children can’t recognize or respond to them. Conjugate vaccines, a special type of subunit vaccine, get around this problem.
When making a conjugate vaccine, antigens or toxoids from a microbe that an infant’s immune system can recognize are linked to the polysaccharides. The linkage helps the immature immune system react to polysaccharide coatings and defend against the disease-causing bacterium. The vaccine that protects against Haemophilus influenzae type B (Hib) is a conjugate vaccine. |
|
|
What is the mode of action for DNA vaccines?
|
DNA vaccines are generally injected into muscle or skin. Encoded antigen is then expressed in myocytes or keratinocytes. For activation of T cells, antigen must be transferred to a 'professional' antigen-presenting cell, usually a dendritic cell (DC). This indirect process of transfer of antigenic material, possibly as apoptotic vesicles, is termed cross-presentation. A small proportion of DNA is also taken up directly by DCs and the encoded antigen can then be processed and presented endogenously.
|
|
|
Why are recombinant vector vaccines so helpful? What can be used as vectors? How are these vaccines made? Which diseases are these trying to protect against?
|
Recombinant vector vaccines are experimental vaccines similar to DNA vaccines, but they use an attenuated virus or bacterium to introduce microbial DNA to cells of the body. “Vector” refers to the virus or bacterium used as the carrier.
Portions of the genetic material from pathogenic microbes are inserted into the genomes of certain harmless or attenuated viruses. The carrier viruses then ferry that microbial DNA to cells. Recombinant vector vaccines closely mimic a natural infection and therefore do a good job of stimulating the immune system. Attenuated bacteria also can be used as vectors. In this case, the inserted genetic material causes the bacteria to display the antigens of other microbes on its surface. In effect, the harmless bacterium mimics a harmful microbe, provoking an immune response. Researchers are working on both bacterial and viral-based recombinant vector vaccines for HIV, rabies, and measles. |
|
|
What are plasmid DNA vaccines? How are they desgined? Why are these important?
|
These are designed so that a stronge ptomoter ddrives the expression of one or more genes encoding proteins of interest.
The immunigenicity of DNA plamids promised to revoluatione vaccine development. Eliminated roadblocks to vaccine dvelopment: pathogen isolation, growth, purification, attenuation Protein identification, prodcution, and purification Tools of meolceular biology used to isolate/clone relevant ganes Animal studies indicate that DNA vaccines can induce protective antibody and CTL responses in vivo |
|
|
Compare Live, Killed, and DNA vaccines in terms of production, boosters, stability, type of immunity induced, and reversion tendency.
|

|
|
|
What is the recommended schedule for vaccination of children?
|
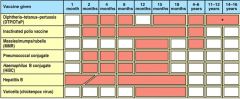
|
|
|
What is herd immunity? How does it work?
|
The term “herd immunity” describes immunity that occurs when the vaccination of the majority of the population (or herd) provides protection to un-vaccinated individuals.
Although no vaccine offers 100% protection, the spread of disease from person to person is much higher in those who remain unvaccinated. The critical percentage of vaccinated individuals to effectively stop a disease depends on the disease and the vaccine. In many instances, however, it is around 90%. |
|
|
What are different types of adjuvants and their respective delivery systems?
|
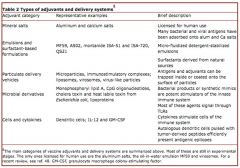
|
|
|
What is the H1N1 vaccine? What are some considerations with it?
|
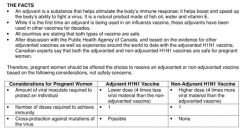
|
|
|
Describe adjuvants for DNA vaccines? What is the charge? How does it bind?
|
The microparticle is cationic because of the presence of the cationic detergent hexadecyltrimethyl ammonium bromide (CTAB), which binds to the surface during microparticle preparation, and allows the efficient adsorption of poly-anionic plasmids. This approach serves to significantly enhance the potency of the antigens encoded by the adsorbed DNA vaccines.
|
|
|
Cytotoxic granules contain which three major substances?
|
Perforin
Granzymes Serglycin |
|
|
Go through killing of an endogenous antigen.
|
1. Antigen enters cell
2. MHC I heavy chain, aided by calnexin folds 3. beta-microglobulins comes and associate 4. ERP 57 and tapasin proteins will get w/nascient MHC I molecule 5. association w/TAP transporter, forming a peptide-loading complex 6. proteosome will degrade virus into small peptide, which are taken up by TAP transporter 7. peptide-loading complex dissociates 8. MHC I will go through golgi apparatus & present on cell surface 9. CD 8 will recognize, and the cytoskeleton will rearrange. Cytotoxic granules will release perforins, granzymes, serglycins 10. once in, granzymes will cleave BID, which in truncated form binds to mitochondria, releasing cytochrome c into cytosol 11. at the same time, granzymes also activate procaspase 3, which cleaves I-CAD 12. CAD movies into nucleus and degrades DNA, ensuring death |
|
|
What are macrophage markers?
|
CD 14
|
|
|
What are NK cell markers?
|
CD 16, CD56, CD 2
|
|
|
What is the granule independent cytotoxicity?
|
Fas is a transmembrane protein expressed by target cells that belongs to the TNF family
Fas binds to the Fas ligand, which is expressed on CTLs. If a CTL encounters a cell that expresses Fas, the ligand on the CTL will bind to the Fas and activate death signals within the cell. Binding of FasL to Fas causes trimerization of Fas. This initiates a chain of events that ends in the recruitment of pro-caspace 8, which triggers apoptosis. Fas can effect termination of the immune response and is part of the barrier in immune-privileged sites (regions such as the eye that immune cells cannot penetrate). Fas ligand is also expressed by CD4 cells, and is the only known mechanism by which CD4 cells can kill other cells. It is sometimes expressed by tumor cells, and when it does, it counters the granule-independent cytotoxicity mechanism (by making the Tc cells kill themselves). |
|
|
What is Canale-Smith Syndrome?
|
a mutation in Fas that results in an inability to perform Fas-mediated cell death. Lymphocytes and Igs are elevated, and the patients exhibit immune hyperactivity that leads to various autoimmune reactions. Hemolytic anemia, caused by autoimmune antibodies directed against RBCs is typical.
|
|
|
Recap TCR activation.
|
1. The TCR recognizes the MHC+peptide+ copreceptors
2. ITAMs are phosphorylated by LCK (attached to the CD8) 3. ZAP70 attaches to the phosphorylated TCR and is itself phosphorylated 4. ZAP70 phosphorylates LAT and SLP-76, which forms a complex together 5. The LAT/SLP-76 complex recruits PLCγ 6. PLC gamma eventually cleaves PIP2 into i. DAG 1. Recruits and activates other kinases which deliver additional activation signals ii. IP3 1. Opens Ca channels, to increase intracellular Ca levels |
|
|
What do NK cells secrete?
|
mainly IFNγ and TNFα
|
|
|
How does the Nef protein work in HIV?
|
HLA A and B but not HLA C. HLA C is the variant recognized by NK cells that sends the NK cells inhibitory signals. Thus, downregulation of HLA A and B means that the infected cell can't be regulated by T cells. By leaving HLA C alone, the virus achieves downregulation of NK cells.
|
|
|
How is the humoral response divided in terms of phases? What is produced in these stages?
|
1. Primary--IgM is produced. Because IgM is a pentamer, it is particularly effective.
2. Secondary--enhanced production of IgG |
|
|
Go through the 4 stages of B cell maturation. What activates each step?
|
1. Generation in bone marrow
2. Elimination of B cells that react against self-tissue 3. Foreign antigens activate B cells in lymph nodes 4. Differentiation into plasma or memory cells The early stage of B cell development in the bone marrow is controlled by communication between the bone marrow stroma and the stem cell that will give rise to the B cell. 1. VLA 4 on the stem cell binds to VCAM on a stromal cell, and causes the stem cell to differentiate into an early pro-B cell 2. Kit on the stem cell binds to SCF (stem cell factor) on the stromal cells, causing the early pro-B cell to express the IL7 receptor, and differentiate into a late pro-B cell. 3. IL 7 binds to IL7 receptors on the developing cell and causes the late pro-B cell to differentiate into a pre-B cell. IL7 makes the pre-B cell i. Downregulate concentrate of its adhesion molecules, allowing it to detach from the stroma ii. Mature and proliferate 4. IL 7 also causes a pre-B cell differentiates into an immature B cell. Immature B cells express IgM. |
|
|
Talk about gene rearrangement in terms of B cell differentiation.
|
1. Specific copies of V, D, and J are rearranged to form the heavy chains
2. Specific copies of V and J are rearranged to form the light chains In the… 1. Early pro B cell--the D and J segments of the heavy chain are rearranged 2. Late pro B cell--the V, D, and J segments of the heavy chain are rearranged 3. Pre B cell--the V and J segments of the light chain are rearranged. Pre B cells also have surrogate light chains, which differentiate into either kappa or lambda light chains, by the time the cell matures into an immature B cell Immature B cells express IgM. Mature B cells express IgD and IgM. |
|
|
What are the two types of B cells? What do they express? How are they important? What do they require?
|
1. B1 cells--express CD5, a marker usually associated with T cells, and are produced in the fetus, arising from stem cells during early embryonic life. In the adult, they are the major B cell type in the peritoneal and pleural cavities. B1 cells can produce antibodies spontaneously, mostly IgM. They do not require T cells to be activated.
2. B2 cells--regular, CD4-expressing B cells, present after birth. They do not produce antibodies spontaneously, and produce mostly IgG. They require T cells. |
|
|
How are B cells activated? Go through this activation process.
|
On the cytoplasmic domain of the BCR, there is an ITAM (immunoreceptor tyrosine-based activation motif), that is part of the α and β chains. ITAMs can interact with several tyrosine kinases.
1. Binding of an AG to the receptor leads to phosphorylation of the ITAM 2. Phosphorylation of the ITAM leads to activation of many intracellular proteins, via more phosphorylation, including: i. Phosphorylation of BTK (Brouton's tyrosine kinase), with BLNK as a helper molecule 1. In X-linked agammaglobulinemia, the BTK is deficient, and the patient is immunodeficient ii. Active BTK activates PLCγ2 iii. Signals are activated which lead to proliferation and differentiation of the B cell |
|
|
How is BCR regulated? How is it inhibited? What do these receptors contain?
|
The BCR can be enhanced by the participation of positive co-receptors. There are three positive co-receptors:
1. TAPA 1 2. CD2 (same thing as CD21) 3. CD19 Co-receptors bind to the AG and enhance the ITAM phosphorylation cascade BCRs can also be inhibited by co-receptors, including: 1. FcγRIIb 2. CD22 3. PIgRB These inhibitory receptors contain ITIMs (immunoreceptor tyrosine-based inhibition motifs) that activate phosphatases that inhibit B cell activation and proliferation. ITIMs are a major target for therapies against B cell malignancies or autoimmune diseases. |
|
|
What is T cell independent activation? How many are there? How do they work?
|
1. TI 1--LPS, activates mature and immature B cells. A high concentration of TI 1 antigens results in a non-specific response, and a low concentration of TI 1 antigen results in an antigen specific response.
2. TI 2--polymeric proteins or capsule polysaccharides, cross-links with the mIg receptor. With the help of cytokines, TI 2 antigens activate mature B cells and inactivate immature B cells. Only TI 2 antigens can signal B cells to produce IgM. Then, activated dendritic cells can release a cytokine called BAFF (B cell activating factor) that signals B cells to: i. Produce more antibodies against the class 2 antigen ii. Undergo antibody class switching and produce IgG |
|
|
What is true for somatic cells?
1. All have identical DNA 2. All have identical RNA 3. 1 and 2 are incorrect 4. 1 and 2 are correct |
1,2 are incorrect.
Immune cells rearrange their receptor genes. |
|
|
On exposure to a new virus, what is most correct?
1. A general immune response will occur 2. Antigen on the virus will be recognized by specific receptors on pre-existing immune cells 3. The virus will stimulate the production of cells that can mount a specific immune response 4. There will be no immune response |
Antigen on the virus will be recognized by specific receptors on pre-existing immune cells. Immune cells in the body have the capacity to recognize particular Ags, even before they encounter that Ag.
|
|
|
What are RAG1 and RAG2?
|
Specific enzymes called RAG1 and RAG2 (recombination activating genes) control VDJ recombination. These enzymes are encoded for by transposable elements of the DNA that probably originated from viruses.
RAG1 and RAG2 recognize specific sequences called recombination signal sequences (RSSs), arranged so that the enzymes "know" whether the gene being recombined comes before or after the signal sequence. |
|
|
Go through V and J recombination?
|
The V and J gene segments recombine first. During recombination:
1. RAG binds to the RSS after the selected V segment, and before the selected J segment 2. Cleavage of the DNA 3. Optional insertion of random nucleotides in the DNA break, by an enzyme called TdT 4. Repair of the DNA by regular dsDNA break repair enzymes, to place the randomly selected V next to the randomly selected J |
|
|
What type of rearrangement occurs in late proB cell?
|
Heavy chain rearrangement is completed. If a cell that rearranged one of its chromosomes did not end up with a gene sequence that could code for a functional protein (ex: because it stuck in a stop codon too early), the other chromosome undergoes rearrangement. Thus, each heavy chain gets two chances to produce a functional protein.
If the second rearrangement is non-productive, the cell will undergo apoptosis. |
|
|
What type of rearrangement occurs in preB cell?
|
VJ, light chain rearrangement occurs. Rearrangement of both the kappa and the lambda genes occurs, on one chromosome if productive, and then on the other chromosome, if the first rearrangement was non-productive.
|
|
|
Talk about primary immune response and somatic hypermutation and switch recombination.
|
In the primary immune response, mostly mediated by IgM, AG/AB affinity is not very high. Then, the antibody undergoes a process called somatic hypermutation, which improves the AB/AG affinity. After undergoing somatic hypermutation, the antibodies undergo a process called switch recombination, in which the heavy chain, that defines the AB as G, M, E, etc. is exchanged.
Immunization with a single antigen will result in a monoclonal response. However, the monoclonal response will give rise to many different antibodies, due to mutations in the hypervariable region. The process of VDJ recombination of T cells happens in the thymus and bone marrow. The process of improving AB/AG affinity after cells encounter the AG happens in the spleen and lymph nodes, particularly, in the germinal centers. B cells can also undergo a process called switch recombination, in which one class of antibody is exchanged for a different class (e.g. A for M, E, G, etc). This process is also mediated by AID. When AID introduces mutations in the "switch region," the result is a DNA break and deletion of the intermediary sequence. |
|
|
Describe B cell differentiation and proliferation in the germinal centers. What happens there? What else is there?
|
B cells that have encountered antigens enter the dark zone of the germinal center. Cells proliferate in the dark zone, creating a clonal group of B cells. Then, specific mechanisms cause lots and lots of mutations to occur in the rearranged gene, resulting in the production of many different proteins--this process is called somatic hypermutation, and happens in the V segment of the gene.
Some of the proteins produced as a result of these mutations will be non-functional, and the cell will die. Most will give rise to a less effective AB. A small percent will give rise to a more effective AB, with higher AG affinity. Dendritic cells sit in the light zone of the germinal center, presenting antigens on their surfaces. The clones that have generated the best antibodies compete with each other for binding to the dendritic cells. Cells that bind the DCs receive survival signals. Cells that do not bind the DCs undergo apoptosis in the light zone. The presence of lots of apoptotic cells is what makes the light zone light. |
|
|
What is Activation induced cytidine deaminase?
|
Activation induced cytidine deaminase (AID) is an enzyme that's critical for the somatic hypermutation process. This enzyme is localized to the nucleus of immune cells. It removes cytidine from the DNA and replaces it with uridine. This results in the substitution of an A for G (because A binds U).
This point mutation causes a DNA break, and then a subsequent DNA repair. Sometimes the repair is successful, and sometimes it is not, leading to a high rate of mutation. A protein called BCL6 provides active inhibition of the DNA repair system. It's not understood why this process is confined to the hypervariable regions but it is the mechanism that makes the hypervariable regions hyperly variable (Note to the English Dept: hyperly is not a real word). |
|
|
What is Hyper IgM syndomre?
|
B cells can also undergo a process called switch recombination, in which one class of antibody is exchanged for a different class (e.g. A for M, E, G, etc). This process is also mediated by AID. When AID introduces mutations in the "switch region," the result is a DNA break and deletion of the intermediary sequence.
|
|
|
Why is somatic hypermutation in the germinal centers dangerous?
|
Somatic hypermutation in the germinal centers is a very dangerous process, and can lead to mutations in protooncogenes that result in lymphomas.
99.9% of cells in the germinal centers undergo apoptosis, during which the DNA is chopped up. If a cell is "rescued" in the beginning of apoptosis, the high rate of mutations and recombinations can often lead to cancer. Hodgkin's is an example of such a cancer. Hodgkin's cells are B cells that have mutations making their Igs non-productive, which suggests that these cells were supposed to undergo apoptosis after their (normal) hypermutation was non-productive, and "by mistake," didn't. A large percentage of Hodgkin's cells contain the Epstein Barr virus, which stops apoptosis. |
|
|
What is the difference b/w a primary immunodeficiency and a secondary?
|
A primary immunodeficiency is a condition resulting from a genetic or developmental defect in the immune system.
A secondary/acquired immunodeficiency is the loss of immune function due to some external environmental cause.occurs when the immunodeficiency is not the main problem, but is the result of some other defect or disease. Examples of patients who will develop secondary immunodeficiencies include patients with: 1. CF 2. HIV--the genes of an infected patient are no different from a non-infected person 3. Nephrotic syndrome, due to loss of immune components 4. Patients on medications that suppress the immune system (ex: chemo) 5. Asplenia--removal of the spleen, for example, due to an accident, would lead to an immunodeficiency 6. Sickle cell |
|
|
What is X-linked agammaglobulinemia?
|
This disease usually manifests as recurrent bacterial infections in boys, between 4-12 months. Patients can also often develop meningitis.
X linked agammaglobulinemia is caused by a block in B cell maturation, due to a mutation in the btk gene, which is important in B cell maturation. Patients will have a low B cell count, with no cells expressing the CD19 B cell marker. Lack of B cells also means a lack of antibody production. Patients lack lymphatic tissue and tonsils. Bone marrow biopsies show the arrest of B cell maturation. |
|
|
What is SCID? What happens? What are the mechanisms that result in T cell deficiency?
|
SCID is the most severe immunodeficiency. If patients are not diagnosed early, the disease could easily be fatal. SCID typically presents at a young age.
The US has instituted neonatal SCID testing, in order to diagnose patients as early as possible. SCID patients always have defective T cells. Then, depending on where in T cell development the deficiency is, the B cells and NK cells may also be deficient. The four major mechanisms that lead to T cell deficiency in SCID are: 1. Premature death of T cells, due to toxicity (deficient T, B, and NK cells) 2. Defective cytokine-dependent survival signals (deficient T and NK cells) 3. Defective VDJ rearrangement (deficient T and B cells) 4. Defective pre-TCR and TCR signaling (deficient T cells--however, although the B cell numbers are normal, they still won't function properly, due to impaired B/T cell interactions) SCID can be caused by a variety of genetic defects. The most common is a defect in the γ chain of the IL2 receptor. The clinical presentation includes failure to thrive, recurrent infections, a lack of lymphoid tissue, and skin, skeletal, and haematological abnormalities. |
|
|
What is a proliferation assay?
|
a test of T cells
1. Cells from the patient are added to a culture and stimulated with antigens or mitogens (materials which stimulate T cell proliferation) to proliferate. 2. Radioactively labeled thymidine is added to the culture. T 3. The cells are allowed to proliferate i. The amount of radioactivity in the culture after the cells are given time to proliferate indicates the amount of new T cells in the culture. The T cells from patients with SCID do not proliferate normally. |
|
|
What is thymus evaluation in terms of SCID diagnosis?
|
Thymus evaluation is also an important part of SCID diagnosis, as T cells undergo their genetic rearrangement in the thymus.
The diversity of the T cell receptors in a patient is an indication of how well the rearrangement process is working in the thymus. A high level of diversity means the process is normal. A low level of diversity indicates abnormal thymus activity. These tests are particularly useful in immunodeficient patients with normal T cell numbers, but abnormal T cell function. |
|
|
What are some common autoimmune diseases?
|
1. Graves
2. Rheymatoid 3. Hashimoto 4. Type 1 Diabetes 5. MS 6. Systemic Lupus |
|
|
What needs to be in place for an AI disease to develop?
|
1. Sufficient clonal expansion of the autoreactive T cells
2. Induction of a functional phenotype that makes a T cell population pathogenic (i.e. cytokines that will allow the autoreactive cells to proliferate) 3. Access of the activated, autoreactive T cells to the target organs (e.g. in multiple sclerosis, the autoreactive T cells need to enter the brain for the disease to manifest) 4. Sufficient expression of MHC molecules and co-stimulatory molecules on APCs in the target area 5. A defect in the expression of regulatory cells 6. Inappropriate MHC expression |
|
|
What are some mechanisms for autoimmunity?
|
1. A cell that undergoes somatic hypermutation does not get deleted or become anergic
2. Abnormal receptor editing 3. Epigenetic changes occur |
|
|
Describe epigenetic changes? What are different methods? What is an examples?
|
Epigenetic changes are heritable changes in gene expression that are not due to changes in genome sequence. They are used by the immune system to mount rapid, specific immune responses against pathogens. There are two major examples of epigenetic changes:
1. DNA methylation 2. Histone modification DNA methylation refers to the addition of a methyl group to a CG dinucleotide, catalyzed by DNA methyltransferase: Methylation of a particular gene makes it less accessible to transcription factors, and therefore, less likely to be expressed. Histone acetylation is another mechanism of epigenetic regulation. Histone acetylation makes the DNA more accessible to transcription factors, and therefore, more likely to be expressed. Some autoimmune disease are the result of histone acetylation and DNA demethylation that allows for the expression of deleterious genes. Lupus' Disease--factors that inhibit DNA methyltransferase such as hydralazin and procainamide block DNA methylation of CD4 T cells, which facilitates the autoimmune reaction of the disease. Tumor cells use DNA methylation and histone deacetylation to block the expression of tumor suppressor genes. |
|
|
What are the various mechanisms whereby an infection can result in an autoimmune disease?
|
1. Molecular mimicry
2. Bystander activation 3. Epitope spread 4. Superantigens |
|
|
What is are three example of molecular mimicry? How do they work?
|
Rheumatic fever--following a strep A infection, molecular mimicry develops between the M protein on the streptococcus and other proteins, expressed on the heart and brain. Autoantibodies begin to attack the myocardium, in the presence of pro-inflammatory cytokines. Cytotoxic T cells are triggered to destroy myocardial and valvular tissue.
NB: molecular mimicry is not the same thing as cross reactivity. Molecular mimicry occurs when an autoreactive cell or antibody recognizes and destroys a self-structure on a pathogen or autoantigen. Cross reactivity occurs when an autoreactive cell or antibody simply recognizes a self-structure on a pathogen or autoantigen. Multiple sclerosis--an autoimmune disease of the CNS. In MS patients, myelin basic protein and myelin oligodendrocyte glycoprotein act as self-antigens and are attacked by macrophages and T cells. The T cell response in MS may be the result of a hep B infection, since hep B viral polymerase is very similar to MBP. According to this hypothesis, hep B polymerase induces a molecular mimicry mechanisms which triggers the T cells to start destroying host tissue. Guillain-Barre syndrome--an immune-mediated disease of the PNS, in which autoantibodies attack the myelin sheaths and axons of peripheral nerves. In many cases of Gullain-Barre, the disease follows an acute infection of the campylobacter jejuni bacterium. This infection results in the production of the autoantibodies that attack the myelin sheaths. One possible mechanism for this disease is that a lipooligosaccharide (LOS) on the bacterial cell wall is also present on the myelin sheaths, resulting in molecular mimicry. |
|
|
What is bystander activation? How does it work? What is an example?
|
Bystander activation is another mechanism whereby an infection can result in an autoimmune disease. It refers to the activation of T cells against a particular antigen during an immune response generated against another antigen.
For example, under normal circumstances, cytotoxic CD8 T cells are regulated by cells called T regulatory cells, which act to inhibit the cytotoxic cells. If the T regulatory cells are impaired, target cells will get killed without control of the TC cells. In such a case, an infection in one site may activate unregulated T cell killing in one site that may migrate and cause tissue destruction in another site. The ability of activated TC cells to attack tissue apart from the infection site may be granted by cross reactivity. For example: In the cases of a latent EBV infection, B cells will be infected. These B cell will be resistant to apoptosis, and may migrate to different sites of the body and cause different infections, depending on where they end up: If they end up in the… They cause… Thyroid Autoimmune thyroiditis Salivary glands Sjogren's syndrome Brain Multiple sclerosis Autoimmune disease following an EBV infection can occur if the cytotoxic T cells that are specific to EBV antigens "mistakenly" fail to recognize and destroy an infected B cell. In such an event, the infected cell will survive. Normally, autoreactive T cells are meant to undergo apoptosis. However, if an autoreactive T cell encounters an EBV infected B cell, it will receive co-stimulatory survival signals. This will cause the bad T cell to survive, proliferate, and go on to attack host tissue. |
|
|
What is systemic lupus erthyematous? How does it work?
|
SLE is a multisystemic disease that primarily affects the kidneys and brain, and involves the generation of more than 150 autoantibodies to various cellular components.
Lupus is caused by the development of macrophages and immature dendritic cells to autoantigens derived from apoptotic cells. Under normal conditions, cells that undergo apoptosis as part of healthy tissue turnover are supposed to be cleared from the body. In Lupus, these cells are not cleared. Enzymes involved in the process of apoptosis such as caspaces cause mutations in these cells which lead to express novel proteins, called neoantigens. Thus, the body gains a population of circulating, failed-apoptotic cells expressing neoantigens that have the capacity to generate an immune response. SLE also involves activation of a toll-like receptor, called TLR9, which helps trigger the autoimmune response. Activation of TLR9 results in the formation of immune complexes on pre-dendritic cells. Activated B cells develop into plasma cells, without any inhibitory feedback response. The main mechanism of pathology in SLE is not primarily the defect in clearing apoptotic cells, but the formation of immune complexes that results from the high concentration of antibodies circulating in the blood, due to the expression of the neoantigens. These complexes activate complement, which results in the production of more antibodies, and more complexes. Then, the complexes aggregate in the kidneys and eventually destroy the glomeruli. The main cause of death in SLE patients is glomerular nephritis. |
|
|
What happens in Hashimoto's?
|
antibodies bind to thyroid peroxidase and block the production of thyroid hormones, resulting in hypothyroidism.
|
|
|
How can rheumatoid arthritis be treated?
|
Rheumatoid arthritis can be treated by IL1 and TNFα blockers.
Remicade and humira are anti-TNFα drugs that are used to treat rheumatoid arthritis. Enbrel is another drug, made from an antibody that blocks binding of TNFα to its FcRII receptor. |
|
|
What is pemphigus vulgaris? How does it work?
|
--antibodies against a molecule called desmoglein, that normally helps anchor the epidermis to the dermis are produced, causing detachment of the epidermis and blister formation on the skin and on internal linings.
Babies of women with pemphigus vulgaris may also develop the disease, due to transfer of the autoantibodies through the placenta. Pemphigus vulgaris is treated wth plasmapheresis (the exchange of blood), IVIg, and immunosuppressants. |
|
|
What is Wegener's granulomatosis? How does it work?
|
an inflammation of the small blood vessels that typically affects the upper respiratory tract and kidneys. This disease is characterized by the presence of anti-neutrophil cytoplasmic antibodies that activate neutrophils, causing oxidative bursts the result in the destruction of the endothelium.
|
|
|
What are TLRs?
|
Recognize bacteria's cell wall. Leads to activation of TF NFxB and expression of inflammatory genes.
|
|
|
TLR 1, 2, 6
|
PDG
Lipoproteins Mycobacteria Yeast |
|
|
TLR 3
|
dsRNA
|
|
|
TLR 4
|
LPS
Lipoteichoic acids |
|
|
TLR 5
|
Flagellin
|
|
|
TLR 7
|
ssRNA
|
|
|
TLR 8
|
G-rich oligionucleotides
|
|
|
Explain the mechanism for TLR 4?
|
In order for it to be expressed on the right space of the membrane, it needs MD2, which directs it to the membrane. LPS is problematic b/c it’s hydrophobic; therefore TLR 4 does not recognize it immediately. LPS will be bound to LPS binding protein and transfer it to CD14 THEN it will be delivered to TLR4.
Once it is activated, there are two pathways it could go through. (Don’t need to know the signal tranduction pathways, just eventually, it will activate NFxB). Pathways: MyD88-dependent or independent. |
|
|
Describe Phase I of neutrophil recruitment.
|
Locallization -- macrophages secrete cytokines = vasodilation. Slowing down of blood causes margination, allowing for endothelial-WBC interactions.
|
|
|
Describe Phase II of neutrophil recruitment.
|
Once leukocytes marginate, rolling & adhesion.
1. Induction of adhesion molecule on endothelium more sticky 2. Weak binding mediated via selectins and will slows the WBC by rolling. P-selectins are made de novo and bind to carbs. 3. Strong binding mediated via integrins (LFA 1), which cause firm adhesion. Expressed on leukocutes. |
|
|
How do integrins function in "rolling and adhesion?"
|
Integrins are heterodimers of alpha and beta chains. Firm adhesion will only occur after conformational change of integrins. Though constantly expressed by leukocutes, they are expressed in inactive form. Dur to cytokines and chemokines (lie CXCL8) will bind to receptor, and integrin will become activated and undergo conformational change.
|
|
|
What is LAD?
|
Leukocyte Adhesion Deficiency: Defect in beta2, recurrent infections, impaired wound healing. (integrin problems)
Neutrophils cannot be recruited to site of infection. If there is no full stop, they cannot get out of vessel. |
|
|
What are the soluble factors of the innate immune response?
|
Complement factors, collectins, defensins, acute phase response proteins.
|
|
|
What are the soluble factors of the adaptive immune response?
|
Immunoglobulins, cytokines
|
|
|
What is the advantage of the form of the neutrophil nucleus?
|
It allows for the cell to squeeze through endothelial cells to reach site of infection and cause local inflammation
|
|
|
What are the two main lymphatic channels?
|
The right lymphatic duct, and the thoracic duct.
|
|
|
The right lymphatic duct drains?
|
Drains the right side of head and neck, right arm, right thorax. Empties into right subclavian vein.
|
|
|
The thoracic duct drains what?
|
Drains the rest of the body. Empties into the left subclavian vein.
|
|
|
What are some developmental landmarks for the thymus?
|
It is at its maximum size at puberty, and gets smaller at maturity.
|
|
|
What is the function of the thymus?
|
Differentiation and maturation of T-cells
|
|
|
What are responsible for the production of antibodies?
|
B-lymphocytes. They are specific and can attack antigens that are invading our bodies.
|
|
|
The major function of this cell is to identify cells that are infected by a virus and destroy them.
|
T-Cytotoxic lymphocytes.
|
|
|
On a T-cell receptor, where is the constant domain located? The variable domain?
|
The constant domain is the membrane anchor, the N-terminus region is the variable domain
|
|
|
What is clonal selection?
|
The antigenic determinant triggers the lymphocytes that recognize it.
|
|
|
What are CD molecules?
|
Cluster of differentiation molecules. Membrane bound molecules whose responsibility is to help in cell to cell recognition and contact.
|
|
|
Which type of T-cell is found more in circulation?
|
The T helper cell in generally a 2:1 ratio to the T-cytotoxic cell.
|
|
|
What are CD3 molecules?
|
Present in all T-lymphocytes. These molecules are sitting very close to the T-cell Receptor and they are helping the T-cell receptor send the signal to the nucleus of the triggered T-lymphocytes.
|
|
|
What makes up MHC class I?
|
Made up of one polypeptide chain coded by a gene and another small molecule (B2 microglobulin) which is coded by a different gene.
|
|
|
What makes up MHC class II
|
Is made up of α and β polypeptide chains.
|
|
|
What are the important MHC Class I genes?
|
HLA-A, HLA-B, HLA-C
|
|
|
What are the important MHC Class II genes?
|
HLA-DQ, HLA-DR, HLA-DP
|
|
|
What chromosome are MHC genes found on?
|
Chromosome 6
|
|
|
What happens when a clone is triggered?
|
Initially we have a relatively small number of cells in a clone. Lymphocytes must differentiate and proliferate.
|
|
|
What are the 4 stages of the immune response?
|
1.Recognition of the antigen by specific antigen receptor
2.Clonal selection, cells differentiate and multiply to increase clone of cells 3.elimination of the pathogen and production of antibodies 4.Production of memory cells |
|
|
What is Phase III of neutrophil recruitment?
|
Extravasation.
Leukocytes pass through endothelial cells. Requires integrins LFA-1 (to ICAM I) and Mac 1 and CD 31. Crossing is called diapedesis. |
|
|
What is Phase IV of neutrophil recruitment?
|
Chemotaxis.
Movement according to gradient. In order for a cell to be chemoattracted, the cell must express relevant chemokine receptors. |
|
|
What is CXCL8 (IL-8)?
|
Chemokine that mobilizes, activates, and degranulates nuetrophils.
|
|
|
What is CCL2 (MCP-1)?
|
Activates macrophases and basophil histamine release. Promots T(h)2 immunity.
|
|
|
Explain the mechanism of signaling via chemokine receptors.
|
1.Chemokine receptor is 7-TM spanning receptor
2.Associates with Large G complexa subunit + GDP/GTP binding domain beta subunits 3.Binding of a chemokine results in association of the receptor with the G complex 4.GDP is exchanged with GTP (activation of complex) 5.Alpha subunit + GTP are separated from beta unit 6.alpha-GTP and beta associate with other adaptors and signal 7.After signaling GTP is exchanged with GDP and the inactive G complex is restored |
|
|
HIV
|
affects CD4, impairs neutrophil funtion. CCR5 is chemokine receptor.
|
|
|
Describe septic shock.
|
1.Body tissues do not receive sufficient oxygen
2.Caused during disseminated uncontrolled infection 3.Could be a complication of a local infection (primarily gram negative bacteria) 4.High mortality 5.Occurs mainly as a result of “cytokine storm,” which is an extreme form of immune reason 6.Dominant cytokine is TNF, IL-1, and IL-6 are important 7.Signs & symptoms could be mostly explained by high systemic effects of TNF |
|
|
What is IL 1?
|
Aka. LAF, MCF.
Receptors for IL-1 found on T cells, B cells, leukocytes, and many other cells. |
|
|
What is IL-6?
|
Regulates B and T cell functions; in vivo effects on hematopoiesis. Inducer of acute phase response.
|
|
|
What is IL-12?
|
Important factor in inducing differentiation of T(h)1 subset helper T cells. Also induces interferon gamma production by T cells and NK cells and enchances NK cell activity.
|
|
|
What is the role of B cells as antigen presenters?
|
B-cell receptor can bind a protein, take it in, degrade it, bind it to MHC II and present it and hand it over to a T-cell.
|
|
|
Even with MHC II, the B-cell isn’t active, what must it develop?
|
It needs co stimulatory molecule B7 to be fully activated.
|
|
|
Viral infected cells present to the immune system how?
|
Infected cells take viral proteins and send them to the membrane via MHC Class I
|
|
|
What are the two roles of the APC when triggering an antiviral response?
|
1.Present the antigenic determinant via MHC II to a T-helper cell to make the immune response more efficient.
2.Present antigenic determinant via MHC I to pre-CTL cell. |
|
|
What is the mechanism (short) of MHC Class II
|
Antigenic determinants must come from the outside into endosome, be degraded by lysosomes, identified by Class II molecules, and be shuttled to the membrane.
|
|
|
What is the mechanism (short) of MHC class I
|
The protein must be in the cytoplasm, where proteosomes are. Protein is degraded, shuttled to ER, rescued by MHC class I, shuttled to membrane.
|
|
|
What happens if IL-2 is not functional?
|
Both arms of the immune response will be incapacitated.
|
|
|
What is the main activity of IL-2
|
To cause proliferation and differentiation of T-helper cells. Can also activate other cells such as NK cells, T-cytotoxic cells, macrophages, and B-cells.
|
|
|
What are the two types of T-helper cells and what are the involved in.
|
TH1- cell mediated immunity
TH2- humoral immunity. |
|
|
What secretes IL12? IF-Gamma?
|
Macrophages secrete IL12 and NK cells secrete IF-gamma.
|
|
|
What secretes IL4?
|
TH2 does – positive feedback.
|
|
|
What does TH1 do?
|
It secretes IF-gamma to activate macrophages and NK cells.
|
|
|
What does TH2 do?
|
It releases IL-4,5,6 which accelerate B-cell proliferation and differentiation.
|
|
|
What are the components of the innate response?
|
1. Physical & chemical barriers
2. Phagocytes 3. Lymphocytes 4. Mast cells, basophils |
|
|
What is the sequence of innate immune response elements that a bacteria encounters upon infection?
|
1. Physical or chemical barrier
2. Activation of immune cells 3. Local recruitment 4. Local vascular response 5. Systemic response |
|
|
What are the two pathways in which a bacteria can be killed once it has been ingested by a phagocyte? What diseases are involved with NADPH oxidase and MPO deficiencies?
|
1. O-independent: mediated by antibiotic peptides, enzymes, acids. Less effective.
2. O-dependent: oxidative burst. May or may not depend on MPO. Chronic granulamatous disease, MPO deficiency, respectively. |
|
|
What activates NK cells? What do they secrete?
|
IL 12
INFa/b NK cells secrete y-IFN to kill infected cell |
|
|
What are three major types of PRRs? What are examples of each?
|
1. Non-opsonic:
Mannose Scavenger Receptors for N-formylated peptides TLRs 2. Opsonic: Fc Complement Collectins 3. Cytoplasmic: Nucleotide-binding organization domains (NOD) RIG-1, MDA5 |
|
|
What do TLR 4? What is special about TLR 4?
|
LPS, lipoteichoic acid.
Another protein called MD2, located outside the cell, directs TLR 4 to plamsa membrane. LPS BP binds to LPS and transfers LPS to CD14 -- the complex will now be recognized by TLR 4. Activated TLR-4 triggers a signaling cascade that eventually activates NFkB. |
|
|
What are NOD proteins? How are they like TLRs? What can they do? What types exist? Where are they?
|
Cytoplasmic PAMP receptors (meant to recognize intracellular pathogens). Binding domains similar to TLRs and can activate NFkB.
Can recruit capases. NOD1 -- bind gram neg, in epithelium NOD2 -- bind gram neg & pos, in Paneth cells |
|
|
What are the two categories of chemoattractants?
|
1. Exogenous -- biosynthetic compounds that originate from bacteria. (LPS)
2. Endogenous -- signals originating from the infected tissue that help recruit leukocytes to the inflamed area. (complements, CXCL8, CCL2, etc) |
|
|
What do chemokines play a role in?
|
1. Angiogenesis
2. Metastisis of cancerous cells 3. Inflammation and immune cell recruitment 4. Would healing = trafficking of many cell types |
|
|
What is CCL2? What does it do? Where is it expressed?
|
Chemokine expressed on macrophages, monocytes, fibroblasts. Activates macropages and promotes histamine release from basophiles and Th2 immunity.
|
|
|
Where is CCX8 expressed? What does it do?
|
Expressed on neutrophils and naive T cells. Mobilizes, activates, and degranulates neutrophils, and stimulates angiogenesis.
|
|
|
What is important about CCR5? CCR7?
|
CCR5 -- chemokine receptor that acts as a co-receptor for HIV infection
CCR7 -- chemokine receptr that helps dendritic cells migrate from the site of infection to a nearby lymph node |
|
|
In context of inflammation, what are cytokines involved in?
|
1. Moblization and migration of cells/
2. Systemic and organ-related effecs on tissues 3. Mediation of active immune response |
|
|
What are the major inflammatory cytokines?
|
IL-1
IL-6 TNFa |
|
|
What does IL-1 do?
|
Activates vascular endothelium, lymphocytes, local tissue destruction, access of effector cells.
|
|
|
What does TNFa do?
|
Activates vascular endothelium, increases vascular permeability, leading to increased entry of IgG complement, and cells to tissues, and increased fluid drainage to lymph nodes.
|
|
|
What does IL-6 do?
|
Lymphocyte activation, increased AB production.
|
|
|
What causes septic shock?
|
Excess concentrations of cytokines (TNFa and IL-6), leading to vasodilation and vessel leakiness = decreased oxygenation.
|
|
|
What does IL-1 do?
|
1. Upregulates adhesion molecules
2. Acivates T cells 3. Induces acute phase reactants 4. Synergizes with TNFa 5. Endogenous pyrogen |
|
|
What does IL-6 do?
|
1. T and B cell growth and differentiation
2. APR and fever |
|
|
What does IL-12 do?
|
Induces CD4 Th differentation into Th1 cells and NK activation
|
|
|
What does TNFa do?
|
1. Activates macrophages, PMNs, and Tc cells
2. Induces PMN-endothelial cell adhesion 3. Causes sepsis, cachexia, pyrexia, acute phase proteins 4. Tumor cell lysis |
|
|
What does IFNy do?
|
1. Induces class I and II MHC
2. Stimulates differentation of monocues into macrophages 3. Activates macrophages 4. Inhibits Th2 cytokines |
|
|
How are T lymphocytes migrated and differentiated in the body?
|
T lymphocytes leave bone marrow, migrate to the thymus. Undergo several process proliferation and differentiation into either helper or CTL.
Produce Ag receptors in thymus and identify antigenic determinants |
|
|
What is contained in the various pulps in the spleen?
|
1. Red -- erthrocytes
2. White -- clusters of lymphocytes |
|
|
What are they different MHC molecules? How do they vary? Where are they expressed? What do they activate?
|
MHC I -- composed of 1 poplypep chain, to which a beta microglobulin binds. Present on all cells of the body. Activate CTL.
MHC II -- composed of 2 polypep chains, alpha & beta. Only on Ag presenting cells. Activate helper cells. |
|
|
What do MHC I molecules presents?
|
Endogenous peptides (viruses)
|
|
|
What do MHC II molecules present?
|
Exogenous (bacterial)
|
|
|
How are antigens recognized?
|
B cells recognize Ag determinants in any form, but need help from cytokines produces by T helper cells.
T lymphocytes respond to peptidic antigens presented by MHC molecules. Dendritic cells recognize Ags using PRRs. |
|
|
What is the difference in binding of epitopes b/w B cells and T cells?
|
B can bind the epitope directly whether or not it is on an APC.
T cannot recognize an epitope if it is not on an APC. |
|
|
What are the four different types of molecules needed to activate T lymphocytes?
|
1. Phagocytotic
2. TLR 3. MHCs 4. Co-stimulatory molecules |
|
|
What are dendritic cells? What do they contain? How do they work?
|
They phagocytose and degrade pathogens. MHC II mocucules wil bind Ag fragments and move to the surface. Cell with then migrate to lymph, where Ag will be presented to T cells.
|
|
|
What to CTLs recognize?
|
MHC I molecules, present in all cells but not in APCs. This is how it recognizes what should be killed.
|
|
|
Where are CD3 molecules expressed? What are the involved with?
|
CD3 molecules, expressed on the membrane of T cells, forms a complex with TCRs that acts as the antigen-binding receptor.
Initiates a cascade of molecular events inside the cells that eventually lead to cell proliferation and cytokine production. |
|
|
What does T cell activation require?
|
T cell receptor /HCM (APC)
CD28 (T)/ B7 (APC) |
|
|
Go through the reaction of T helper cells and APC interactions.
|
1. APC presents Ag via MHC II
2. CD4 molecule on T lymphocyte recognizes MHC II and helps bind (T) ___ (APC) CD2/LFA-3 LFA-1/ICAM TCR/MHC II CD28/B7 |
|
|
How is an MHC I assembled?
|
1. Class I heavy chain is stabilized by calnexin until b2-microglobulin binds
2. Calnexin is released and the heterodimer of class I heavy chain and b2m forms a complex with calreticulin, tapasin, an TAP. 3. A peptide delivered by TAP binds to the class I heavy chain, forming the mature MHC class I molecule. 4. The class I molecule dissociates, exported to ER |
|
|
What types of cytokins to APCs secrete? Which 3 signals are sect to the T cells?
|
1. Chemokines (mediate chemotaxis)
2. Cytokines (T cell differenttion) - IL-6 - IL-12 - TGF-b |
|
|
What do Th1 cells do? What do they produce? Where are the receptors for this secretion? What is the structure?
|
Activates Tc cells. Produces IL-2.
IL-2 receptor, which are present on T cells, are composed of 3 polypep Presence of a-chain indiciates that the T cell have activate. The gamma chain can be mising in a genetic mutation, which will case SCID. |
|
|
What do Th2 cells do?
|
help B cells proliferate and differentiate.
|
|
|
Which interleukins are involved in the differentiation and proliferation of B cells?
|
IL-4, IL-5, IL-6
|
|
|
Which IL is involved with Ig isotype switching (IgE)?
|
IL-4
|
|
|
What IL promotes the isotype (IgA)?
|
IL-5
|
|
|
What does IL-10 do?
|
Inhibits synthesis of Th1-type cytokines
Down-regulates class II expression |
|
|
What does IL-12 do?
|
Promotes cytolytic activity
Macrophage activation |
|
|
B27
|
psoriasis
ankylosing spondylisis IBD Reiter's |
|
|
B8
|
Graves
|
|
|
DR3
|
DM type I
|
|
|
What is LAD type I?
|
a) defect in LFA-1 integrin (CD18) protein on phagocytes
b) presents as recurrent bacterial infections, absent pus formation, delayed separation of umbilicus |

