![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
3 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
What mass of nitrogen is contained in a 57m³ tank if the pressure and temperature are 1 atm and 21°C respectively? |
66.1 kg |
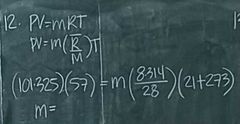
|
|
|
A 0.71 m³ tank contains 4.5kg of an ideal gas. The gas has a molecular weight of 44 and is at 21°C. What is the pressure of the gas? A. 352.2 kpa B. 325.2 kpa C. 532.2 kpa D. 523.2 kpa |
A. |
|
|
|
A volume of 450 cm³ of air is measured at a pressure of 740 mm Hg absolute and a temperature of 20°C. What is the volume in cm³ at 760 mm Hg absolute and 0°C
A. 516.12 B. 408.25 C. 620.76 D. 375.85 |
B. |
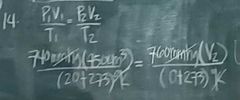
|

