![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
45 Cards in this Set
- Front
- Back
|
Can charge molecules be aromatic?
|
Yes!
|
|
|
When it comes to charge stabiliziatoin, which is more powerful: resonance stabilization or inductive stabilization?
|
resonance stabilization
|
|
|
Aldehyde shows up where on the H NMR?
|
9.8ppm sharp
|
|
|
Addition of Amine to Carbonyl Group
|
Primary amine ==> Imine
Secondary amine ==> Enamine (Note: tertiary and quaternary amines do not react with the C=0 group.) |
|
|
E2 reaction will only occur with what conformation?
|
antiperiplanar conformation
|
|
|
What is the most straightforward method of making a benzoic acid from benzene?
|
- Place a methyl group and then oxidize the methyl group to the carboxylic acid...
- Can do this with AlBr3/CH3Br to get the methyl group on; and then oxidize with KMnO4 |
|
|
How do you make bromobenzene from benzene?
|
FeBr3/Br2
|
|
|
Reacting an alcohol with PCC will give you what?
|
a ketone
|
|
|
A ketone that reacts with a primary amine give an _____
|
imine
|
|
|
What is an imine?
|
a functional group or chemical compound containing a carbon-nitrogen double bond
|
|
|
(T/F) Whenever there is a double bond, there exists the possibility of having geometric isomers.
|
True
|
|
|
What happens when you react imines with lithium aluminum hydride (LAH)
|
create an amine... LiAlH4 is a reducing agent b/c there are a lot of H's attached to either B or Al.
|
|
|
If you obtain two products of unequal yield, what does this suggest?
|
that hte two products are diastereomers.
|
|
|
Whenever you see one molecule goign to form a ring, what should you suspect?
|
intramolecular reaction
|
|
|
What is faster: intramolecular or intermolecular reactions?
|
intramolecular reactions
|
|
|
If charged intermediates are involved in your mechanism, what should you make sure of?
|
that they go away before you get to the product, unless the product is chraged as well.
|
|
|
Claisen condensation
|
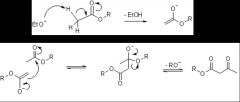
- Step1: At least one of the esters must possess an α-hydrogen atom as the first step of the reaction is deprotonation by the base to give an enolate ion
- Step2: The enolate then attacks the ester giving a β-Keto ester. - Used when have an ester. - Can use NaOMe/MeOH |
|
|
What should you tihnk of whenever a question gives you the pKa of the substance being purified?
|
ion exchange chromatography or isoelectric focusing.
|
|
|
How do you determine the isoelectric points of a sample IN THE CASE OF INDIVIDUAL AMINO ACIDS?
|
In the case of individual amino acids, the isoelectric points can be determiend easily by taking the avg of the two closest pKa values.
|
|
|
Amino acids will be ______ charged at pH values below thier pI and ______ charged above their pI.
|
positively; negatively
|
|
|
How do you determine the isoelectric points of a sample IN THE CASE OF A PROTEIN when you have the individual pI of the amino acids?
|
FIND ANOTHER METHOD.
*** DO NOT TAKE THE AVERAGE OF THE pI OF THE INDIVIDUAL AMINO ACIDS! |
|
|
What should you tihnk of whenever a question gives you the pKa of the substance being purified?
|
ion exchange chromatography or isoelectric focusing.
|
|
|
How do you determine the isoelectric points of a sample IN THE CASE OF INDIVIDUAL AMINO ACIDS?
|
In the case of individual amino acids, the isoelectric points can be determiend easily by taking the avg of the two closest pKa values.
|
|
|
Amino acids will be ______ charged at pH values below thier pI and ______ charged above their pI.
|
positively; negatively
|
|
|
How do you determine the isoelectric points of a sample IN THE CASE OF A PROTEIN when you have the individual pI of the amino acids?
|
FIND ANOTHER METHOD.
*** DO NOT TAKE THE AVERAGE OF THE pI OF THE INDIVIDUAL AMINO ACIDS! |
|
|
ANILINE
is this an organic/inorganic/base/acid? |
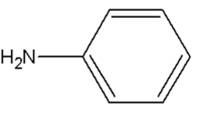
organic compound and weak base
|
|
|
acetophenone / methyl phenyl ketone
- properties? |
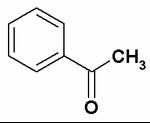
hydrophobic since none of functional groups are capable of making hydrogen bonds.
|
|
|
phenol
organic/inorganic/basic/acidic |
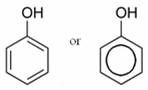
organic and weak acid.
|
|
|
Is the organic phase always on top of the aqueous layer during an extraction?
|
no. It depends on the density!
|
|
|
During extraction, how do you transpose a compound that is acidic or basic to the aqueous phase?
|
add base or acid, respectively.
|
|
|
Nitrile
|

~2200 cm^-1 indicates presence of a triple bond
(alkyne or nitrile) |
|
|
what is an anion exchanger?
|
something that attracts anions and thus must have a positive charged group.
|
|
|
What can column chromatography be used for?
|
to separate substances based on size or charge.
|
|
|
How does an eluent for a column chromatography work?
|
- they must be eluted based on their affinities for the stationary phase. For this purpose, NaCl is added in an increasing concentration gradient.
- the least negatively charged proteins will emerge from the column before the proteins with the greatest negative charge. |
|
|
All forms of chromatography have two ____
|
phases: a stationary phase and a mobile phase.
- separations of compounds take advantage of the different affinities that compounds have for the two different phases. |
|
|
What should you watch out for when dealgin with chromatography?
|
Be cautious not to assume that the desired product will attach to the stationary phase. You may be presented with a sitaution in whcih purification will be better accomplished if the desired product has a higher affinity for the mobile phase.
|
|
|
For NMR spectrum, the number of signals you see tells you a lot about what?
|
the symmetry ==> the fewer signals there are, the higher symmetry the molecule possesses.
|
|
|
If get a sibnglet, this signifies what?
|
that there are no protons on adjacent carbons.
|
|
|
IR stretch at 1740 cm^-1 indicates?
|
presence of carbonyl.
|
|
|
IR stretch at 2220 cm^-1 indcates?
|
presence of a triple bond (either an alkyne or a nitrile)
|
|
|
In order fora substance to have a carboxylic acid, it must have what kind of IR stretch.
|
Alcohol
|
|
|
If a stretch is closer to 1740 than to 1700, it is probably ____
|
an ester
|
|
|
If the stretch is closer to 1700 than to 1740, it is probably a _____
|
ketone
|
|
|
If a molecule has four or more sites of unsaturation, what should you immediately suspect?
|
aromatic ring is present
|
|
|
Sites of Unsaturation equation
|
(2C + 2 -H)/2
|

