![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
35 Cards in this Set
- Front
- Back
|
What are metabolic pathways? |
A series of interconnected biochemical reactions which convert a substrate into a product through a step wise process each catalysed by an enzyme |
|
|
Using a diagram, describe anabolic and catabolic pathways, explaining clearly how they are linked. |
Anabolic synthesizes large complex molecules from small simple ones. Requires ATP. Catabolic breakdown later complex molecules into smaller simpler molecules. Releases ATP. |
|
|
Define an amphibolic pathway and give an example |
A pathway which can either be catabolic or anabolic depending on either the availability of energy or indeed the need for energy. Ex. TCA cycle |
|
|
What is the function of key junction points? |
To allow metabolites to enter into alternative pathways when required. |
|
|
Give 3 key junctions, their 3 sources of molecules and their 3 fates. |
Glucose-6-phosphate - glucose in glycolysis, pyruvate, glycogen - pyruvate, glycogen, ribose-5-phosphate Pyruvate - glucose-6-phosphate, alanine, lactate - lactate, transamination to alanine, carboxylation to oxaloacetate Acetyl CoA - glycolysis, beta oxidation of fatty acids, ketogenic amino acids - CO2 by TCA cycle, fatty acid synthesis, ketogenesis |
|
|
How can metabolic pathways be regulated by the presence of compartments? |
Because the fates of certain molecules depends on where they are located within the cell e.g. if they are in the cystol or the mitochondria. |
|
|
Give an example of regulation by compartments. |
Fatty acids are transported into the mitochondria for oxidation only when energy is required. Fatty acids remaining in the cystol are esterified or exposed. |
|
|
3 advantages of a multienzyme complex. |
Concentration of reactions in one location Protection of intermediates Increased catalytic efficiency Prevention of unwanted side effects |
|
|
Give an example of reciprocal regulation of the glycolytic pathway |
Glycolysis - glucose to pyruvate Gluconeogenesis - pyruvate to glucose |
|
|
Name the components of ATP |
Adenine organic base, ribose sugar, three inorganic phosphates. |
|
|
Participation of ADP in muscle cells by the enzyme creatine kinase can provide a useful boost to the supply of ATP. Describe in words this chemical equation and state why it has so useful. What is this process an example of? |
Phosphocreatine + ADP -> creatine + ATP Useful at times when demand is high, such as during intense exercise Substrate level phosphorylation |
|
|
What chemical process does the enzyme adenylate kinase catalyse? |
Catalyses the reversible transfer of phosphate group from one ADP molecule to another to generate ATP and form AMP |
|
|
Epinephrine (adrenaline) cannot pass through the phospholipid bilayer of the cell membrane and therefore must exert it's effect through a signal transition cascade. Describe the role of G proteins in this process |
Epinephrine binds to a GPCR (G protein couples receptor) located in the plasma membrane. GTP replaced GDP on the G protein- it becomes active The G protein activates the effector- adenylyl cyclase |
|
|
Name second messenger produced in G protein process |
cAMP |
|
|
The second messenger diffused through the cytoplasm and activates other enzymes called kinases, the first being protein kinase A. What is the function PDF kinases? |
Kinases are class of enzymes that phosphorylate other proteins and so alter their activity. |
|
|
What is the end effect of epinephrine in the cells of skeletal muscle? |
The activation of glycogen phosphorylase enzyme which breaks down glycogen into glucose ( provides energy for the fight or flight response) |
|
|
In a "fed state", insulin is released by the beta cells in the Islets of Langerhans. Describe two effect of this increased insulin on skeletal muscle tissue. |
Increase in GLUT4 transported in the plasma membrane. Increased glucose uptake into cells |
|
|
What is the effect of insulin on liver cells? Which enzyme is involved in this process? |
Conversion of glucose into glucose-6-phosphate and then into glycogen Glycogen synthase |
|
|
Using a diagram, describe the homeostatic process involved when glucose levels increase and also fall |
G |
|
|
What is the reaction catalysed by glycogen phosphorylase? How is the enzyme regulated? |
Glycogen phosphorylase activity is controlled by reversible phosphorylation and dephosphorylation Glycogen phosphorylase removes glucose units from glycogen |
|
|
Activity of some enzymes is altered as a result of binding of a molecule - an allosteric regulator or effector - to the enzyme at a site other than active site Using a diagram, explain how downstream metabolites in a pathway can alter enzyme activity. |
A -|-> B --> C --> D --> E -->F ^ | |_______________________| F is a negative allosteric effector of the enzyme covering A to B Molecule F binds to the regulator site The affinity of the active site for molecule A is diminished. |
|
|
Name a positive modulator of the glycolytic enzyme phosphofructokinase. |
ADP |
|
|
Why is the pyruvate dehydrogenase complex a major regulatory point for metabolism in cellular respiration? |
It is the lining reaction between glycolysis and the citric acid cycle |
|
|
Where is the pyruvate dehydrogenase complex located? |
The mitochondrial matrix |
|
|
Reducing power in the form of either NADH or FADH2, is generated at which of the following metabolic stages? |
Glycolysis The pyruvate dehydrogenase complex The citric acid cycle |
|
|
Which membrane transporter protein is responsible for translocating pyruvate across inner mitochondrial membrane? |
Pyruvate translocase |
|
|
Name the three enzymes that comprise the large multi-enzyme complex PDC. |
E1 - pyruvate dehydrogenase E2 - dihydrolipoyl transacetylase E3 - dihydrolipoyl dehydrogenase |
|
|
Which overall reaction does the PDC catalyse? |
Pyruvate to Acetyl coA and carbon dioxide |
|
|
The pyruvate to Acetyl CoA and CO2 can be broken down into three chemical transformations, requiring all three enzymes in the complex. Name them. |
Decarboxylation Oxidation of carbonyl group to carboxyl group Activation by linkage to CoA through a thioester bond |
|
|
The PDC channels substrates from over enzyme to another. how is the 2-carbon metabolic substrate kept attached to the substrate? |
By the prosthetic groups |
|
|
What two mechanisms control the PDC? |
Product injunction by NADH and Acetyl CoA Covalent modification by phosphorylation of the E1 subunit |
|
|
Using a diagram, highlight the possible source and fate of the following molecules: Glucose-6-phosphate, pyruvate and Acetyl CoA. |
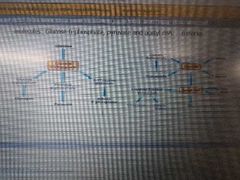
|
|
|
The production of ATP in the cristae of the mitochondria using the process of chemiosmosis is called oxidative phosphorylation because of the involvement of oxygen in the process. Briefly describe this process. |
NADH and FADH2 carry H and higher energy electrons to the ETC located in the cristae of the mitochondria. H+ are pumped into the intermembrane space - creating a H+ gradient High energy electrons are passed along protein carried complex H+ ions pass through ATP synthase generations ATP from ADP+Pi High energy electrons, H+ ions and oxygen combine to form water |
|
|
Give two examples of substrate level phosphorylation seen glycolysis. |
Conversion of 1,3-bisphosphateglycerate to 3-phosphoglycerate Conversion of phosphoenolpyruvate to pyruvate |
|
|
Give two examples of substrate level phosphorylation seen glycolysis. |
Conversion of 1,3-bisphosphateglycerate to 3-phosphoglycerate Conversion of phosphoenolpyruvate to pyruvate |

