![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
9 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Strong acids |
HCl, HBr, HI, HClO3, HClO4, H2SO4, HNO3 |
H ion plus non metal |
|
|
Strong base |
Any group 1 and 2 metals with OH ion |
|
|
|
Single bond |
Sigma |
Sigma or pi? |
|
|
Size of atom/metallic character/atomic radius trend |

|
They are all the same |
|
|
Ionization energy/electronegativity |
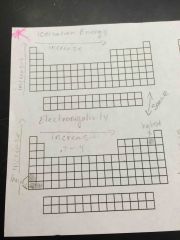
|
|
|
|
Electron affinity trend |
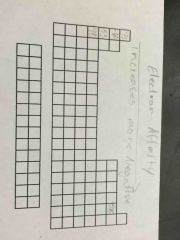
Increasingly more negative |
|
|
|
Zeff trend |
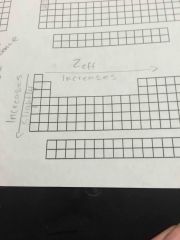
|
|
|
|
Double bond |
1 pi and 1 sigma |
Sigma or pi |
|
|
Triple bond |
2 pi and 1 sigma |
Sigma or pi |

