![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
30 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
What are the five major groups of fixatives? |
1.Aldehydes 2.Mercurials 3.alcohols 4.oxidizing agents 5. Picrates |
|
|
|
What are the two types used in aldehyde fixatives? |
Formaldehyde (formalin) and glutaraldehyde |
|
|
|
Formaldehyde (formalin): |
Fixed by cross linkages formed in proteins especially between lysine residues does not harm structure of proteins so antigenicity is not lost thus good for immunohistochemical techniques penetrates tissue well BUT slow standard solution for LM is 10% neutral buffered formalin. buffer prevents acid is the acidity that would promote auto lysis and cause precipitation of formal-heme pigment in tissues |
|
|
|
Glutaraldehyde |
Causes the deformation of alpha helix structures and proteins so it’s NOT good for immunohistochemical staining fixes very quickly so good for EM penetrates very poorly but gives the best overall cytoplasmic a nuclear detail Standard solution is 2% buffered glutaraldehyde |
|
|
|
While the standard solution for light microscopy is 10% neutral buffered formalin, we fix the entire eye by using _____ percent because 10% formalin quickly fixes the sclera but the process reduces ________ |
5% permeability of the sclera and prevents good fixation of intraocular tissues |
|
|
|
Mercurials |
Fixes tissue by unknown mechanism contain mercuric chloride and includes such well-known fixatives such as B5 and Zenkers Penetrate poorly and cause tissue hardness but are fast and give excellent nuclear detail primarily used to fixate hematopoietic and reticuloendothelial tissues Bc they contain mercury they must be disposed of carefully due to the toxicity. rarely used |
|
|
|
Alcohols |
Alcohols including methyl alcohol and ethyl alcohol (ethanol) are protein denaturants which causes brittleness and hardness Good for cytologies smears Because they act quickly and give a good nuclear detail Spray cans of alcohol fixatives are marketed to clinicians doing PAP smears or conjunctival scrapings but cheap hair sprays do just as well! |
|
|
|
Oxidizing agents |
Include permanganate fixatives (potassium permanganate), dichromate fixatives (potassium dichromate),and osmium tetroxide They cross-link proteins but cause extensive denaturation Osmium is used mostly an EM because it adds contrast and definition to membranes |
|
|
|
Picrates |
Include fixatives with picric acid among these is Bouin’s sol’n Unknown mechanism It does almost as well as mercurials with nuclear detail but does not cause as much hardness. picric acid is an explosion hazard in dry form as a solution, it stains everything it touches yellow including skin |
|
|
|
In order to cut thin sections of tissue, it must be embedded in a support matrix using either |
Paraffin or plastic resins |
|
|
|
Paraffin or plastic resin’s, however, or not water soluble so tissue must be processed through a series of chemicals that will allow the water and tissue to be ____ |
replaced with the support media |
|
|
|
So then tissue processing is a series of 4 major steps |
1. Rinse fixative out with running water 2. Dehydrate In graded series of ethanol concentrations (50%, 70%, 95%, 100%) 3. Clear specimen in an organic solvent that is miscible with alcohol and embedding medium. This step washes lipids out of tissues so other methods are required if lipids must be preserved 4. Infiltrate specimen using combo of clearing agent and embedding medium |
|
|
|
Embedding (2 steps) |
1. Orient specimen in embedding capsule 2. fill embedding medium ( paraffin or resin with accelerator) |
|
|
|
Sectioning (for paraffin sections) What’s used? |
A microtome is used with a very sharp steel blade. Ribbons of sections 5 to 8 µm thick are produced |
|
|
|
Ultra thin sectioning. What’s required? What’s used? |
Plastic embedding is required to obtain very thin sections needed for EM cut with diamond knives and laid off onto a tiny water bath for pick up with copper grids |
|
|
|
Frozen sections. When are these used?(2) |
1. When intraoperative decisions are required 2. When lipids must be preserved for staining |
|
|
|
Collecting paraffin sections |
Collect it on slides Then put on a warmer that melts sections onto slide |
|
|
|
Staining paraffin sections |
Most of the dyes are water soluble to stain the tissue the process used to replace water with embedding medium must be REVERSED!!! Done so by putting slides into a series of baths. Xylene dissolves paraffin and is miscible with 100% ethanol Slides are then processed in progressively dilute ethanol’s (100%, 95%, 70%, 50% and then water) After staining the process must be reversed one more time to ensure the sections are permanently mounted. The water must now be replaced with mounting medium, the transparent material that will permanently hold cover slip over the stained sections. |
|
|
|
How to replace with mounting medium (after staining (water soluble dyes)) |
Slides are run back through baths of ethanol (50%, 70%, 95% and then 100%) to dehydrate followed by clearing with xylene and coating in mounting medium finally the cover slip is placed an allowed to dry |
|
|
|
What are the most common stains used? (3) |
1. Hematoxylin and eosin H&E 2. Periodic acid-Schiff PAS 3. Masson/Mallory Trichrome |
|
|
|
What was used to stain this tissue? |
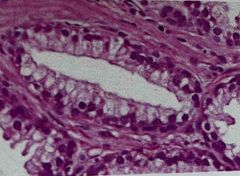
Back (Definition) |
|
|
|
Hematoxylin & Eosin (H&E) |
Hematoxylin is basic and thus acidophilic (acid loving) nuclei contain nucleic acid which turn blue with Hematoxylin. it also stains keratohyalin granules in the skin and calcific deposits Eosin (is acidic and thus basophilic-base loving )is used as the counterstain which stains virtually everything pink EXCEPT nuclei |
|
|
|
What was used to stain this tissue? |
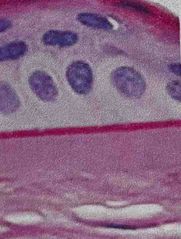
Back (Definition) |
|
|
|
Period acid- Schiff (PAS) |
Used to detect polysaccharides such as glycogen and muco-substances Essentially useful for identifying TRUE basement membranes (PAS+) you’ll see magneta color Counterstain light purple for nuclei |
|
|
|
What was used to stain this tissue? |
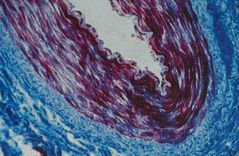
Back (Definition) |
|
|
|
Masson/Mallory Trichrome |
Permits separating muscle fibers FROM connective tissue fibrils of collagen are blue Fibroglia, neuroglia and muscle fibers are red fibers of elastin are pink or yellow Ex) medium artery: Smooth muscle stained red, surrounding connective tissue blue |
|
|
|
What are two special stains for neural tissues? |
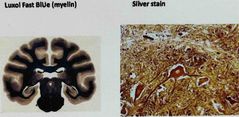
1.Luxol fast BLUe (myelin) 2. Silver stain |
|
|
|
What special stain was used for these tissues? |

Back (Definition) |
Elastic tissue stain & Alcian blue stain (mucins like goblet cells) |
|
|
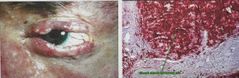
What special stain is used for sebaceous gland carcinoma or frozen sections? |
Oil red O |
|
|
|
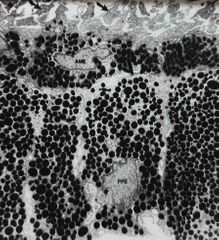
Staining for TEM (transmission electron microscopy) |
Sections remain in plastic Once collected on grids, the whole grid is stained with uranyl acetate and lead citrate which put metals into membranes to provide contrast in scope |
|

