![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
125 Cards in this Set
- Front
- Back
|
Describe the 3 ionic currents which underlie a cardiac action potential.
|
Inward Na+ is the initial current, causing rapid depolarisation.
Inward Ca2+ is responsible for the lengthy plateau phase (slow hyperpolarisation) Outward K+ rapid hyperpolarisation at the end of the AP |
|
|
Why is calcium important in cardiac muscle contraction?
|
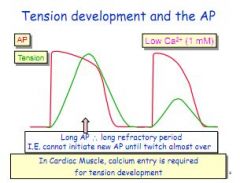
Calcium entry generates the plateau, creating a longer depolarisation period, allowing the cardiac muscle to achieve greater tension. Less calcium means shorter refractory period means less strong muscle contraction.
|
|
|
Describe the path of depolarisation in the heart.
|
Sinoatrial node > across atria (R and L) > Atrioventricular node > bundle of His > Left and right bundles > Purkinje fibres
|
|
|
Describe the AP in the SA node?
|
More positive at beginning (-60mV) and decaying, -45mV is start of rapid depolarisation caused by Ca2+ only, NO fast sodium channels, no plateau. Rate of decay from -60 to -45mV determines heart rate.
|
|
|
What determines the heart rate?
|
Decay in the SA node membrane potential.
|
|
|
Does the SA node have rapid Na+ channels, and what effect does this have on the depolarisation?
|
No - so it is slower.
|
|
|
How does the AP differ from the SA node and ventricular muscle?
|
RMP - Ventricular muscle stable -90mV, SAN, decaying -60 to -45mV
Depolarisation - Ventricular muscle, Fast inward Na+, SAN, slow inward Ca2+ Plateau - Ventricular muscle, yes (Ca2+), SAN, No |
|
|
Describe EC coupling in SAN.
|
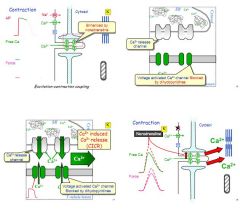
Ca2+ enters cardiac muscle cell via voltage gated channels
Induces Ca2+ release from sarcoplasmic reticulum Ca2+ flood out via L-type Ca2+ channels (CICR) To relax, Ca2+ reuptake by ATP channels back into SR and out via Ca2+-Na+ exchanger and Na+ K+ ATP-ase. |
|
|
How do chronotropic agents alter heart rate?
|
By influencing the rate of decay in the SA node, faster decay means a shorter gap between beats.
|
|
|
Name two chronotropic neurotransmitters, one positively chronotropic and one negative.
|
Positive, NA. Negative, AcH.
|
|
|
Why are low resistance gap junctions important in cardiac muscle?
|
They allow the myocytes to be electrically coupled, so that they act like a electrical synctium.
|
|
|
Describe the relative speed of conductance across the various heart regions.
|
SAN node/atria - 1m/sec
AV node - 0.05m.sec (much slower) Bundle of His, L/R bundles & purkinje fibres - 4m/sec (very fast) Endocardium to epicardium - 0.3m/sec (slow) |
|
|
Place speed of conductance across cardiac tissues in order, fastest first.
|
Bundle of His, L/R bundles & purkinje fibres - 4m/sec
SAN and L/R atria - 1m/sec Endocardium to epicardium (in to out) - 0.3m/sec AV node - 0.05m/sec |
|
|
What is the treppe effect?
|
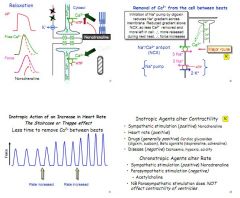
An increase in heart rate brings about an increase in force of contraction (inotropy), thought to be due to the speed of heart reducing the ability of the two pumps to clear intracellular Ca2+ (First is one Ca2+ out for 3 Na+ in, second is 3 Na+ out for 2 K+ in). Increased Ca2+ in the cell causes stronger contraction/.
|
|
|
List 3 positively inotropic agents.
|
Sympathetic nerves (NA)
Heart rate (Treppe effect) Drugs e.g. Cardiac glycosides (Digoxin) or B-agonists (Adrenalin) |
|
|
What effect does disease have on inotropy?
|
Negatively inotropic, e.g. Hypoxia, ischaemia and acidity.
|
|
|
List a positive and a negative chronotropic agent.
|
Positive - NA, acting on B-receptors
Negative - Acetylcholine. |
|
|
List the connection in Einhoven's triangle.
|
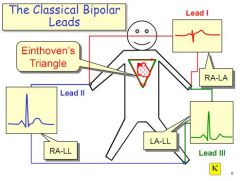
Lead 1 - Right hand to left hand, across top of heart
Lead 2 - Right hand to left foot, along right edge Lead 3 - Left hand to left foot, along left edge. |
|
|
Why does the body act as a volume conductor?
|
We are full of salts which carry a charge.
|
|
|
What is the ECG actually measuring?
|
Extracellular currents between depolarised and resting cells which can be measured at the skin surface.
|
|
|
A positive voltage, i.e. in the same direction as the lead, causes a movement in which direction?
|
Up.
|
|
|
What is a vector quantity?
|
Has both force and direction.
|
|
|
Does the ECG pick up all voltage changes?
|
No. The device sums changes into a single direction, hence although atrial depolarisation moves down and across to the left atria, the ECG displays as a single movement south towards the AV node. Similarly, the ventricles depolarise left and right, but ECG just shows a single movement down the Bundle of His.
|
|
|
Describe the passage of depolarisation and repolarisation in the epicardium and endocardium.
|
Depolarisation - endocardium to epicardium
Repolarisation - epicardium to endocardium (as AP is quicker in epicardium) - so T-wave is 'up' |
|
|
What is septal depolarisation?
|
A very brief L to R depolarisation after conductance through the AV node into the Bundle of His, which causes the left bundle to depolarise just in front of the right.
|
|
|
What are the three augmented leads?
|
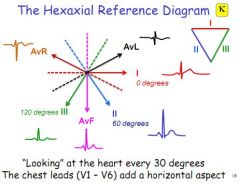
AvR (right shoulder), AvL (left shoulder), AvF (straight down)
|
|
|
What do the 6 precordial leads record?
|
Voltage in the transverse plain, from V1 to V6.
|
|
|
Which direction does ECG pick up best?
|
Parallel. It can detect speed much better in parallel, it is much harder to gauge speed if something is running straight at you.
|
|
|
What are the augmented leads detecting?
|
Voltage differences against the middle - the other two limb leads connected together (assumed to be zero)
|
|
|
What are the bipolar leads detecting?
|
Voltage differences between two points
|
|
|
What is the P-wave?
|
Depolarisation of the atria (it reverses as the atria repolarises)
|
|
|
What is the Q-R-S complex?
|
Q - Brief septal depolarisation left to right (hence negative)
R - Rapid ventricular depolarisation S - Depolarisation along the reverse side of the heart near the base (towards lead 2, hence negative) |
|
|
What is the T-wave?
|
Ventricular repolarisation.
|
|
|
Why are the areas between the P-wave and QRS, and the QRS and T-wave flat?
|
They are isoelectric, the potential is zero (either all depolarised or all at rest).
|
|
|
What causes the P-R interval?
|
Conductance through the AV node, which is slow and take around 0.1 to 0.2 of a second.
|
|
|
What is the S-T interval?
|
The plateau phase of the ventricular muscle contraction.
|
|
|
What might a raised or lowered S-T interval demonstrate?
|

Acute myocardial injury - damage to cells causes them to be partially depolarised/rested at the wrong times, raising or lowering the base line, which will show as a raised or depressed S-T segment.
|
|
|
What might an inverted T-wave demonstrate and why?
|
Delay in conductance so the apex repolarises before the base, which will show up as a negative instead of a positive.
|
|
|
What might a wide P-R interval demonstrate?
|
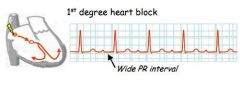
1st degree heart block in the AV node.
|
|
|
Why might you get 2 p-waves for each QRS?
|
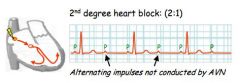
2nd degree heart block, 2:1 ratio means 2 p-waves for each QRS, reflecting the fact that only 1 p-wave is getting through the AVN.
|
|
|
What would you suspect if the P-R interval was getting shorter, then a beat was missed?
|
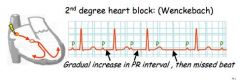
A different kind of 2nd degree block.
|
|
|
Describe 3rd degree block AVN escape.
|
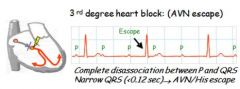
Complete dissociation between P and QRS with a narrow QRS, reflecting AVN excape (current not conducting down Bundle of His)
|
|
|
What is sick sinus syndrome?
|
Loss of occasional SAN rhythm.
|
|
|
Describe 3rd degree block with ventricular escape.
|
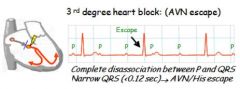
Dissociation between P and QRS, with a wide QRS reflecting ventricular excape.
|
|
|
What might you see with a left bundle branch block?
|

Wide QRS.
|
|
|
What is an arrhythmia?
|
A disturbance in the normal beating of the heart, which can be asymptomatic or life threatening.
|
|
|
What are the four main causes of arrhythmia?
|
Congential (Wolff Parkingson White)
MI (causing ischaemia, tachycardia) Atrial fibrillation (heart failure, dilation, high BP) Idiopathic |
|
|
How are arrhythmias classified?
|

Rate - <60bpm brady, >100bpm tachy
Location - Supraventricular or ventricular Cause - Impulse generation or impulse conductance |
|
|
Describe atrial flutter.
|
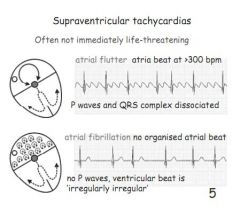
Not life threatening. >300bpm but P and QRS completely dissociated.
|
|
|
Describe atrial fibrillation.
|
Not life threatening. No organised atrial rhythm, no p-waves. Ventricular beat is irregularly irregular.
|
|
|
List two supraventricular tachycardias.
|
Atrial flutter, and atrial fibrillation.
|
|
|
List two ventricular tachycardias.
|
Ventricular tachycardia, and ventricular fibrillation.
|
|
|
Describe ventricular tachycardia.
|
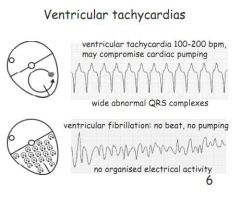
Not necessarily life threatening. 100-200bpm, wide abnormal QRS complexes.
|
|
|
Describe VF.
|
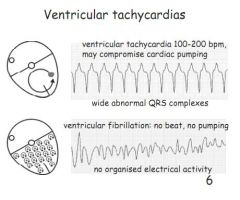
No beat, no pumping, no organised electrical activity. Effectively dead.
|
|
|
What gives rise to the additional beats in some tachyahrrythmias?
|
Other myocardial cells generating alternative APs, which works because of electrical conductance across gap junctions in myocardial cells.
|
|
|
What are the two forms of disorder of impulse formation?
|
Latent pacemakers, and triggered automaticity (after-depolarisations)
|
|
|
What is the main disorder of impulse conduction?
|
Re-entry
|
|
|
Which parts of the conduction system can make potential or latent pacemakers?
|
All of them. Any site outside the SAN node can acquire a pacemaker capability.
|
|
|
Can cardiac muscle cells generate latent beats?
|
Yes, ischaemia can cause muscle cells to develop depolarisation-induced automaticity.
|
|
|
What are EADs and DADs and their causes?
|
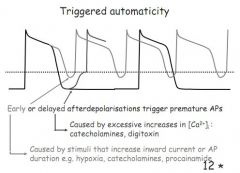
Early After Depolarisations occur during phase 2/3 (plateau/reploarisation) - caused by stimuli that increase inward current or prolong AP duration e.g. hypoxia, catecholamines
Delayed After Depolarisations - occur after phase 4 (at rest) - caused by excessive Ca2+ e.g. digoxin |
|
|
What is re-entry?
|

Impulse is delayed or trapped in a part of the heart. Adjacent tissue repolarises, and is at rest, allowing the trapped current to re-enter. This either is a one-off, or can cause a sustained tachycardia.
|
|
|
What is the most serious arrhythmia after an MI?
|

Re-entry rhythms, either next to damaged cardiac tissue, or congenital.
|
|
|
What are the two types of re-entry?
|
Functional and anatomical.
|
|
|
Describe a functional re-entry.
|
A congenital accessory conductance pathway, as seen in Wolff-Parkinson-White - here conductance occurs through the neighbouring Bundle of Kent, which has a longer refractory period.
|
|
|
What might you see on ECG in a patient with WPW syndrome?
|

An early delta-wave depolarisation by the QRS as the Bundle of Kent depolarises before the AVN.
|
|
|
Other than a congenital cause, what else might generate an anatomical re-entry?
|
A healed MI scar can generate a heart block.
|
|
|
What is a functional re-entry?
|
Occurs when there is no anatomical cause - usually post-MI and caused by a slowed conductance, result can be waves of excitation called rotors - typical in VF.
|
|
|
What is the Vaughan-Williams classification system for arrhythmia drugs?
|
Class 1 - Na+ channel block e.g. lidocaine
Class 2 - B-blocker e.g. propanolol Class 3 - Drugs to prolong the AP e.g. Amiodarone Class 4 - Ca2+ channel blockers e.g. Verapamil |
|
|
What is the main strategy for drug treatment of arrhythmias?
|
Inhibit AP conductance or reduce muscle exciteability.
|
|
|
How do class 1 arrhythmia drugs work and when are they used?
|
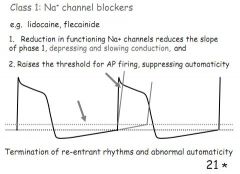
Na+ channel blockers reduce excitability, fewer APs and take longer, phase 1 slowed.
Used to terminate reentrant rhythms and treat abnormal automaticity. |
|
|
How do class 2 ahrrythmia drugs work and when are they used?
|
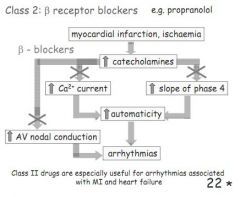
B-blockers, reduce SNS stimulation, lowering Ca2+, reducing decay in phase 4, reduced conductance at the AVN.
Good after an MI. |
|
|
How do class 3 ahrrythmia drugs work and when are they used?
|
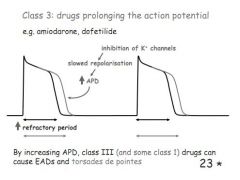
Prolong the AP, inhibit the outward K+ channel, causing a slower repolarisation. Can cause an EAD or a torsades de point.
|
|
|
How do class 4 ahrrythmia drugs work and when are they used?
|
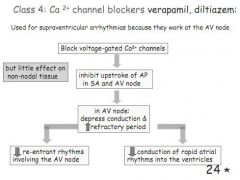
Ca2+ channel blockers, inhibit the AP in the SA and AV node - littel effect on non-nodal tissues. Good for supraventricular rhythms.
|
|
|
What is a good treatment for atrial fibrillation?
|

Digoxin. Stimulates vagal AcH stimulation, slowing heart.
|
|
|
Which drugs control rate of contraction via conductance through the AVN?
|

Rate control drugs: Class 2, B-blockers lower SNS stimulation, less Ca2+. Class 4, Ca2+ channel blockers. Also digoxin which raises [Ca2+] by blocking the Na+K+ ATPase, leading to increased contraction and reduced AV node conductance
|
|
|
Which drugs control the rhythm generated in the SAN and AVN?
|
Class 3, inhibit K+ out so prolong the AP, class 1, block inward Na+.
|
|
|
Why are class 1 drugs not used prophylactically to prevent arrhythmias in post-MI patients?
|
Shown to increase mortality.
|
|
|
What is radiofrequency catherter ablation?
|
Where you destroy an ectopic pacemaker or re-entry site by burning the tissue.
|
|
|
When might you use defibrillation?
|
During VF and pulseless VT. No shock in Pulseless Electrical Activity or in asystole.
|
|
|
Which 3 factors can influence cardiac output?
|
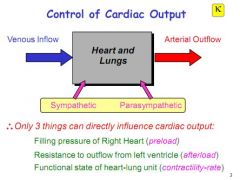
Preload - filling pressure into the right heart
Afterload - resistance to outflow from the left ventricle Contractility/rate - functional state of the heart and lungs |
|
|
Right atrial pressure is equal to central venous pressure, what consequence does this have for stroke volume?
|
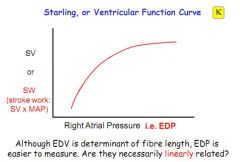
Stroke volume is therefore linked to central venous pressure.
|
|
|
What does the Starling curve demonstrate?
|
That an increase in End Diastolic Pressure (easier to measure than EDV) results in an increase in stroke work (afterload + force of ejection)
|
|
|
What is Starlings law of the heart?
|
That the energy released during heart muscle contraction is dependent on the length of the fibres at the start of contraction.
|
|
|
What the are two reasons why initial length can generate greater contractility?
|
Cross-bridge theory - says that to a point, the greater the stretch, the more powerful the return
Also, the greater the stretch the more sensitive cardiac muscle seems to become to Ca2+ |
|
|
What is credited with the increased sensitivity of muscle to Ca2+ during stretch?
|

Troponin C
|
|
|
Assuming heart rate and contractility are constant, what reflects CO?
|
CVP.
|
|
|
Why is the left ventricle considered less contractile than the right?
|
Thicker, stiffer walls.
|
|
|
What is contractility?
|
Change in force not due to stretch.
|
|
|
Why does left heart failure result especially in pulmonary oedema?
|
Because it can generate very high EDP.
|
|
|
Why is output low in an enlarged heart?
|
La Place's law - surface tension in the smaller heart is greater. In the big heart it is far less.
|
|
|
Does CVP influence venous return?
|
Yes.
|
|
|
Which structures make up the microcirculation?
|
Arterioles, venules and capillaries.
|
|
|
What are the 3 capillary types?
|
Continuous - endothelial monolayer with tight junctions, least permeable
Fenestrated - Kidneys, joints, GI mucosa, very permeable. Sinusoidal - Liver, bone marrow and spleen, big gaps allowing proteins and cells to diffuse across. |
|
|
How do gases move across capillary walls?
|
Lipophilic, so via diffusion. Very quick.
|
|
|
How do small solutes move across capillary walls?
|
Lipophobic, so either via paracellular routes or through fenestrae. Fast.
|
|
|
How does water cross capillary walls?
|
Aquaporins in kidney, but most across paracellular junctions following osmotic gradients.
|
|
|
How do proteins cross the capillary walls?
|
In vesicles. Slow.
|
|
|
How does the BBB affect the passage of molecules into the brain?
|
Very tight junctions, prevents many hydophilic and all hydrophobic substances diffusing across. Gases can diffuse freely, but all other solutes must diffuse via specific transport mechanisms.
|
|
|
How much fluid passes through capillaries each day, and how much moves across capillary barriers?
|
4000L pumped, 80,000 passes across.
|
|
|
Which two forces drive fluid filtration?
|
Hydrostatic and oncotic.
|
|
|
Which solutes have a higher reflection coefficient and what does this mean?
|
Reflection coefficient is determines by the permeability of a substance. If it is less permeable, it has a higher reflection coefficient, and is said to exert greater osmotic pressure.
|
|
|
What additional three factors influence filtration?
|
Closure of arterioles - reduced filtration pressure
In organs where water is reabsorbed, like kidney, proteins are washed out so the oncotic pressure is higher Protein concentration near the permeability barrier is likely to be less than in bulk intersititum. |
|
|
What is the permeability barrier?
|
Glycocalyx.
|
|
|
Are lymph vessels valved?
|
Yes.
|
|
|
Why does oedema develop?
|
Decreased oncotic pressure drawing fluid back into blood.
Blocked lymphatics Increased hydrostatic pressure. |
|
|
Why does inflammation cause oedema?
|

Capillaries become leaky to proteins, interstitium becomes highly osmotic, water drawn out.
|
|
|
Why does starvation cause oedema?
|

Protein in plasma falls, so fluid drawn out of capillaries. This happens most in abdomen where portal system generates highest capillary pressure.
|
|
|
What is heart failure?
|
An inability to provide sufficient CO to supply the needs of the tissues.
|
|
|
When might compensatory mechanisms be insufficient to allow CO and BP to be maintained?
|

In exercise / decompensation.
|
|
|
What is decompensation?
|
When compensatory mechanisms fail, and CO and BP fall.
|
|
|
What causes heart failure?
|

Arrhythmias
Disease in the valves Hypertension Coronary artery disease. |
|
|
Contrast a systolic dysfunction, with a diastolic dysfunction.
|
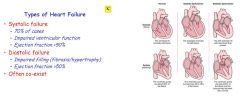
In systolic dysfunction, the ventricles are dilated and enlarged. They fill with more blood, but cannot pump properly, ejecting less than normal.
In a diastolic dysfunction, the ventricles are meaty and stiff, they fill less, so although they pump fine, there is less blood to pump. |
|
|
What are the three types of heart failure?
|
Left failure - most common, caused by ischaemia
Right failure - caused by lung disease, pulmonary hypertension Congestive - when left failure causes right failure |
|
|
What are the common symptoms of heart failure?
|
Tired all the time. Breathless, even at night. Swollen legs, and abdomen.
|
|
|
How does ischaemic heart disease cause left heart failure?
|
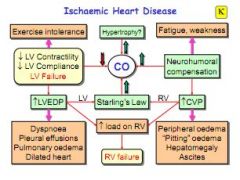
Reduced CO due to raised heart rate, result in bigger heart, reduced contractility and compliance. Resulting in exercise intolerance, raised LVEDP, breathlessness, oedema.
|
|
|
Why does LVEDP go up so much in left heart failure.
|
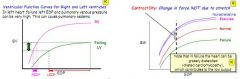
Because you need a massive increase in LVEDP to gain a small increase in stroke volume.
|
|
|
What is a major pulmonary consequence of high LVEDP?
|

Pulmonary oedema due to raised raised hydrostatic pressure.
|
|
|
How does the body compensate in left heart failure, and what are the consequences?
|
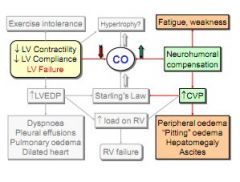
Raised CVP to attempt to raise CO. Result is tiredness due to heart working harder, and peripheral oedema.
|
|
|
What is a side effect of neurohumeral compensatory mechanisms in left heart failure?
|
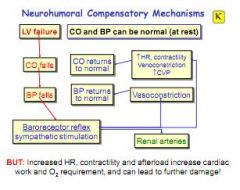
When the LV fails CO falls, BP falls and the baroreceptor reflex kicks in - this drives vasoconstriction, increased HR and contractility, plus venoconstriction. This all restores CO and BP, but the result is more work = further damage
|
|
|
What are the consequences of greater peripheral resistance?
|
Reduced circulation to kidneys and GI, higher BP which can lead to ascites and peripheral oedema.
|
|
|
What causes an enlarged liver?
|
Raised CVP.
|
|
|
How is left heart failure diagnosed?
|
Ejection fraction of <50%.
|
|
|
What are the neurohumoral compensation mechanisms?
|
Baroreceptors can increase SNS stimulation, resulting in increased heart rate and contractility, vaso- and venoconstriction, leading to raised BP and CVP.
|
|
|
What are the renal compensatory mechanisms?
|
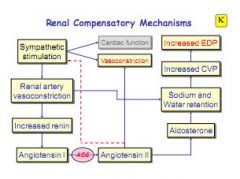
When renal artery is constricted, renin is released, causing increase in Na+ retention and raised blood volume, increasing CVP and EDP.
|
|
|
How does spironolactone, an aldosterone antagonist help in treatment of heart failure and what are the possible side effects?
|
Reduces Na+ retention, lowers blood volume, reduces work of heart. But can cause hypokalaemia and kidney dysfunction.
|

