![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
30 Cards in this Set
- Front
- Back
|
Colour of [Cu(H2O)6] |

Pale blue |
|
|
Colour of [Cu(NH3)4(H2O)2] |

Dark blue |
|
|
Colour of [Cu(Cl)4] 2- |

Yellowish (may appear green due to equilibrium with Cu2+) |
|
|
Colour and state of Cu(OH)2 |

Pale blue ppt |
|
|
CuO |

Black solid |
|
|
CuCO3 |

Green ppt |
|
|
Copper(I) iodide |
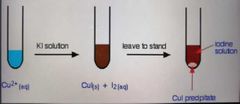
Cream ppt |
|
|
Cu2O |

Brick red ppt |
|
|
Cu2CO3 |

Pale green solid |
|
|
[Fe(H2O)6] 2+ |

Pale green |
|
|
Fe(OH2) |
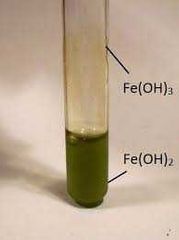
Dirty green ppt (oxidises by atmospheric oxygen to Fe(OH)3) |
|
|
[Fe(H2O)6]3+ |

Yellow |
|
|
Fe(OH)3 |

Reddish brown ppt |
|
|
Mn2+ |

Pale pink(appears colourless at low concentrations usually) |
|
|
MnO2 |

Dark brown ppt |
|
|
MnO4 2- |

Dark green |
|
|
Mn(OH)2 |
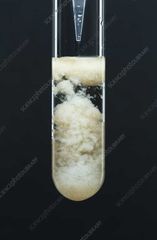
Off-white ppt |
|
|
MnCO3 |

Light brown solid |
|
|
Mn2O3 |

Dark brown solid |
|
|
MnO4 - |

Purple |
|
|
Cr3+ |

Green |
|
|
Cr(OH)3 |
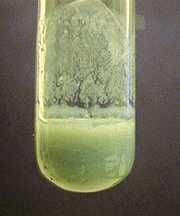
Dirty green ppt |
|
|
[Cr(OH)6] 3- |

Dirty green solution |
|
|
Cr2o4 2- |

Yellow |
|
|
Cr2O7 2- |

Orange |
|
|
V 5+ |

Yellow (may show green intermediate between V4+ and V5+) |
|
|
V 4+ |

Blue (may show green intermediate between V4+ and V5+) |
|
|
V 3+ |

Green |
|
|
V 2+ |

Violet |
|
|
CuCl (sparingly soluble) |

White |

