![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
127 Cards in this Set
- Front
- Back
|
The first step in glycolysis is what? |
The first step in glycolysis is phosphorylation of glucose by a family of enzymes called hexokinases to form glucose 6-phosphate (G6P). This reaction consumes ATP, but it acts to keep the glucose concentration low, promoting continuous transport of glucose into the cell through the plasma membrane transporters. In addition, it blocks the glucose from leaking out - the cell lacks transporters for G6P, and free diffusion out of the cell is prevented due to the charged nature of G6P. Glucose may alternatively be formed from the phosphorolysis or hydrolysis of intracellular starch or glycogen.
Cofactors: Mg2+ |
|
|
The first step in glycolysis is phosphorylation of glucose by a family of enzymes called hexokinases to form glucose 6-phosphate (G6P). Cofactor?
|
The first step in glycolysis is phosphorylation of glucose by a family of enzymes called hexokinases to form glucose 6-phosphate (G6P). This reaction consumes ATP, but it acts to keep the glucose concentration low, promoting continuous transport of glucose into the cell through the plasma membrane transporters. In addition, it blocks the glucose from leaking out - the cell lacks transporters for G6P, and free diffusion out of the cell is prevented due to the charged nature of G6P. Glucose may alternatively be formed from the phosphorolysis or hydrolysis of intracellular starch or glycogen.
Cofactors: Mg2+ |
|
|
Glycolysis (from glycose, an older term for glucose + -lysis degradation) is the metabolic pathway that converts glucose C6H12O6, into what?
|
Glycolysis (from glycose, an older term[1] for glucose + -lysis degradation) is the metabolic pathway that converts glucose C6H12O6, into pyruvate, CH3COCOO− + H+. The free energy released in this process is used to form the high-energy compounds ATP (adenosine triphosphate) and NADH (reduced nicotinamide adenine dinucleotide).
|
|
|
Glycolysis is a definite sequence of how many reactions?
|
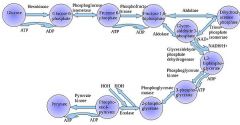
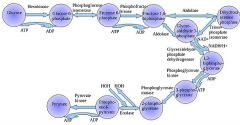
Glycolysis is a definite sequence of ten reactions involving ten intermediate compounds (one of the steps involves two intermediates). The intermediates provide entry points to glycolysis. For example, most monosaccharides, such as fructose, glucose, and galactose, can be converted to one of these intermediates. The intermediates may also be directly useful. For example, the intermediate dihydroxyacetone phosphate (DHAP) is a source of the glycerol that combines with fatty acids to form fat.
|
|
|
The most common type of glycolysis is which pathway?
|
The most common type of glycolysis is the Embden-Meyerhof-Parnas pathway (EMP pathway), which was first discovered by Gustav Embden, Otto Meyerhof and Jakub Karol Parnas. Glycolysis also refers to other pathways, such as the Entner–Doudoroff pathway and various heterofermentative and homofermentative pathways.
|
|
|
The first step in glycolysis is?
|
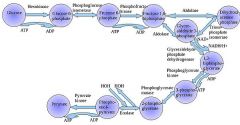
The first step in glycolysis is phosphorylation of glucose by a family of enzymes called hexokinases to form glucose 6-phosphate (G6P).
|
|
|
In glycolysis, G6P is rearranged into what by what?
|
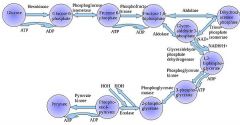
G6P is then rearranged into fructose 6-phosphate (F6P) by glucose phosphate isomerase. Fructose can also enter the glycolytic pathway by phosphorylation at this point.
The change in structure is an isomerization, in which the G6P has been converted to F6P. The reaction requires an enzyme, phosphohexose isomerase, to proceed. This reaction is freely reversible under normal cell conditions. However, it is often driven forward because of a low concentration of F6P, which is constantly consumed during the next step of glycolysis. Under conditions of high F6P concentration, this reaction readily runs in reverse. This phenomenon can be explained through Le Chatelier's Principle. Isomerization to a keto sugar is necessary for carbanion stabilization in the fourth reaction step (below). |
|
|
Draw ten steps of glycolysis.
|
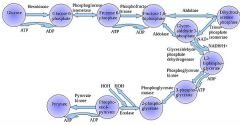
|
|
|
There are over 14 different types of glucose transporter (GLUT) and they allow passage of glucose into the cell by what mechanism?
|
Facilitated diffusion |
|
|
GLUT1 and GLUT3 are located where?
|
GLUT1 and GLUT3 are located in the plasma membrane of cells throughout the body, as they are responsible for maintaining a basal rate of glucose uptake (esp. CNS and RBCs). Basal blood glucose level is approximately 5mM. The Km value (an indicator of the affinity of the transporter protein for glucose molecules; a low Km value suggests a high affinity)of the GLUT1 and GLUT3 proteins is 1mM; therefore GLUT1 and GLUT3 have a high affinity for glucose and uptake from the bloodstream is constant.
GLUT3 is expressed mostly in neurons (where it is believed to be the main glucose transporter isoform), and in the placenta. |
|
|
GLUT2 transporters are located where?
|
GLUT2 in contrast has a high Km value (15-20mM) and therefore a low affinity for glucose. They are located in the plasma membranes of hepatocytes and pancreatic beta cells, and their high Km allows for glucose sensing; rate of glucose entry is proportional to blood glucose levels.
Is a bidirectional transporter, allowing glucose to flow in 2 directions. Is expressed by renal tubular cells, small intestinal epithelial cells, liver cells and pancreatic β cells. Bidirectionality is required in liver cells to uptake glucose for glycolysis, and release of glucose during gluconeogenesis. In pancreatic β-cells, free flowing glucose is required so that the intracellular environment of these cells can accurately gauge the serum glucose levels. All three monosaccharides (glucose, galactose and fructose) are transported from the intestinal mucosal cell into the portal circulation by GLUT2 |
|
|
GLUT 4 transporters are found where?
|
GLUT4 transporters are insulin sensitive, and are found in muscle and adipose tissue. As muscle is a principle storage site for glucose and adipose tissue for triglyceride (into which glucose can be converted for storage), GLUT4 is important in post-prandial uptake of excess glucose from the bloodstream. The drug Metformin phosphorylates GLUT4, thereby increasing its sensitivity to insulin.
|
|
|
GLUT 4 transporter expression is sensitive to what?
|
GLUT4 transporters are insulin sensitive, and are found in muscle and adipose tissue. As muscle is a principle storage site for glucose and adipose tissue for triglyceride (into which glucose can be converted for storage), GLUT4 is important in post-prandial uptake of excess glucose from the bloodstream. The drug Metformin phosphorylates GLUT4, thereby increasing its sensitivity to insulin.
|
|
|
GLUT 5 transporters are found where?
|
GLUT5 is a fructose transporter expressed on the apical border of enterocytes in the small intestine. GLUT5 allows for fructose to be transported from the intestinal lumen into the enterocyte by facilitated diffusion due to fructose's high concentration in the intestinal lumen. GLUT5 is also expressed in skeletal muscle, testis, sprem cells, kidney, fat tissue, and brain.
Fructose malabsorption or Dietary Fructose Intolerance is a dietary disability of the small intestine, where the amount of fructose carrier in enterocytes is deficient.[4] In humans the GLUT5 protein is encoded by the SLC2A5 gene |
|
|
Glycogen is made by which organs / tissues?
|
Glycogen is a molecule that serves as the secondary long-term energy storage in animal and fungal cells, with the primary energy stores being held in adipose tissue. Glycogen is made primarily by the liver and the muscles, but can also be made by glycogenesis within the brain and stomach
|
|
|
GLUT 7 transporters are found where?
|
GLUT7 has been assigned to the class II of the GLUT family on the basis of sequence
similarity, and its closest family member is GLUT5, an intestinal fructose transporter. GLUT7 is primarily expressed in the small intestine and colon, although mRNA has been detected in the testis and prostate as well. The protein is expressed in the apical membrane of the small intestine and colon, and it has a high affinity (0.5 mM) for glucose and fructose. The abundance of the protein in the small intestine does change in parallel with the dietary carbohydrate. However, the distribution of GLUT7 along the small intestine does not entirely match with the availability of glucose and fructose, suggesting that the physiological substrate for this transporter has yet to be identified. |
|
|
Organs capable of gluconeogenesis?
|
Primarily the liver but also the kidney.
|
|
|
Glycogen is a molecule that serves as the secondary long-term energy storage in animal and fungal cells, with the primary energy stores being held where?
|
Glycogen is a molecule that serves as the secondary long-term energy storage in animal and fungal cells, with the primary energy stores being held in adipose tissue. Glycogen is made primarily by the liver and the muscles, but can also be made by glycogenesis within the brain and stomach
|
|
|
Second step in glycolysis?
|
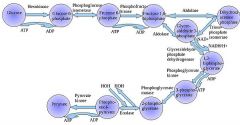
G6P is then rearranged into fructose 6-phosphate (F6P) by glucose phosphate isomerase. Fructose can also enter the glycolytic pathway by phosphorylation at this point.
|
|
|
The first step in glycolysis is phosphorylation of glucose by a family of enzymes called what?
|
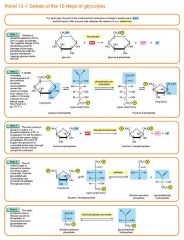
The first step in glycolysis is phosphorylation of glucose by a family of enzymes called hexokinases to form glucose 6-phosphate (G6P). This reaction consumes ATP, but it acts to keep the glucose concentration low, promoting continuous transport of glucose into the cell through the plasma membrane transporters. In addition, it blocks the glucose from leaking out - the cell lacks transporters for G6P, and free diffusion out of the cell is prevented due to the charged nature of G6P. Glucose may alternatively be formed from the phosphorolysis or hydrolysis of intracellular starch or glycogen.
In animals, an isozyme of hexokinase called glucokinase is also used in the liver, which has a much lower affinity for glucose (Km in the vicinity of normal glycemia), and differs in regulatory properties. The different substrate affinity and alternate regulation of this enzyme are a reflection of the role of the liver in maintaining blood sugar levels. Cofactors: Mg2+ |
|
|
Second step of glycolysis: what is rearranged into what?
|
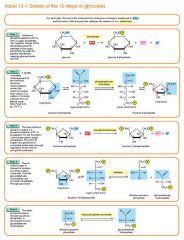
G6P is then rearranged into fructose 6-phosphate (F6P) by glucose phosphate isomerase. Fructose can also enter the glycolytic pathway by phosphorylation at this point.
The change in structure is an isomerization, in which the G6P has been converted to F6P. The reaction requires an enzyme, phosphohexose isomerase, to proceed. This reaction is freely reversible under normal cell conditions. However, it is often driven forward because of a low concentration of F6P, which is constantly consumed during the next step of glycolysis. Under conditions of high F6P concentration, this reaction readily runs in reverse. This phenomenon can be explained through Le Chatelier's Principle. Isomerization to a keto sugar is necessary for carbanion stabilization in the fourth reaction step (below). |
|
|
Second step in glycolysis: G6P is rearranged into fructose 6-phosphate (F6P) by what enzyme?
|
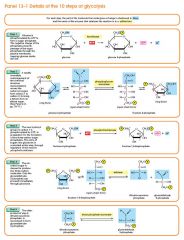
G6P is then rearranged into fructose 6-phosphate (F6P) by glucose phosphate isomerase. Fructose can also enter the glycolytic pathway by phosphorylation at this point.
The change in structure is an isomerization, in which the G6P has been converted to F6P. The reaction requires an enzyme, phosphohexose isomerase, to proceed. This reaction is freely reversible under normal cell conditions. However, it is often driven forward because of a low concentration of F6P, which is constantly consumed during the next step of glycolysis. Under conditions of high F6P concentration, this reaction readily runs in reverse. This phenomenon can be explained through Le Chatelier's Principle. Isomerization to a keto sugar is necessary for carbanion stabilization in the fourth reaction step (below). |
|
|
Third step in glycolysis?
|
Step 3
The enzyme phosphofructokinase uses another ATP molecule to transfer a phosphate group to fructose 6-phosphate to form fructose 1, 6-bisphosphate. Fructose 6-phosphate (C6H11O6P1) + phosphofructokinase + ATP → ADP + Fructose 1, 6-bisphosphate (C6H10O6P2) The energy expenditure of another ATP in this step is justified in 2 ways: The glycolytic process (up to this step) is now irreversible, and the energy supplied destabilizes the molecule. Because the reaction catalyzed by Phosphofructokinase 1 (PFK-1) is coupled to the hydrolysis of ATP, an energetically favorable step, it is, in essence, irreversible, and a different pathway must be used to do the reverse conversion during gluconeogenesis. This makes the reaction a key regulatory point (see below). This is also the rate-limiting step. Furthermore, the second phosphorylation event is necessary to allow the formation of two charged groups (rather than only one) in the subsequent step of glycolysis, ensuring the prevention of free diffusion of substrates out of the cell. |
|
|
Glycolysis step 3
Which enzyme uses another ATP molecule to transfer what to fructose 6-phosphate to form fructose 1, 6-bisphosphate? |
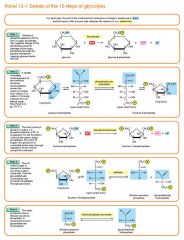
Step 3
The enzyme phosphofructokinase uses another ATP molecule to transfer a phosphate group to fructose 6-phosphate to form fructose 1, 6-bisphosphate. Fructose 6-phosphate (C6H11O6P1) + phosphofructokinase + ATP → ADP + Fructose 1, 6-bisphosphate (C6H10O6P2) |
|
|
Glycolysis step 3
The enzyme phosphofructokinase uses another ATP molecule to transfer a phosphate group to what to form what? |
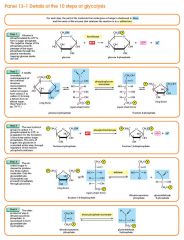
Step 3
The enzyme phosphofructokinase uses another ATP molecule to transfer a phosphate group to fructose 6-phosphate to form fructose 1, 6-bisphosphate. Fructose 6-phosphate (C6H11O6P1) + phosphofructokinase + ATP → ADP + Fructose 1, 6-bisphosphate (C6H10O6P2) |
|
|
Glycolysis step 4
Which enzyme splits fructose 1, 6-bisphosphate into what? |
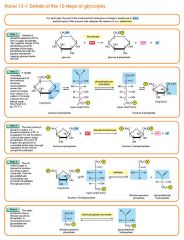
Step 4
The enzyme aldolase splits fructose 1, 6-bisphosphate into two sugars that are isomers of each other. These two sugars are dihydroxyacetone phosphate and glyceraldehyde phosphate. Fructose 1, 6-bisphosphate (C6H10O6P2) + aldolase → Dihydroxyacetone phosphate (C3H5O3P1) + Glyceraldehyde phosphate (C3H5O3P1) |
|
|
Glycolysis step 4
The enzyme aldolase splits what into what? |
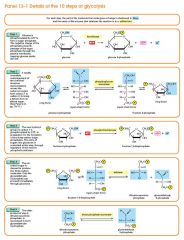
Step 4
The enzyme aldolase splits fructose 1, 6-bisphosphate into two sugars that are isomers of each other. These two sugars are dihydroxyacetone phosphate and glyceraldehyde phosphate. Fructose 1, 6-bisphosphate (C6H10O6P2) + aldolase → Dihydroxyacetone phosphate (C3H5O3P1) + Glyceraldehyde phosphate (C3H5O3P1) |
|
|
Glycolysis step 5
The enzyme triose phosphate isomerase rapidly inter-converts the molecules dihydroxyacetone phosphate and glyceraldehyde phosphate. Glyceraldehyde phosphate is removed as soon as it is formed to be used in the next step of glycolysis. Dihydroxyacetone phosphate (C3H5O3P1) → Glyceraldehyde phosphate (C3H5O3P1) Net result for steps 4 and 5: Fructose 1, 6-bisphosphate (C6H10O6P2) ↔ 2 molecules of Glyceraldehyde phosphate (C3H5O3P1) |
Glycolysis step 5
The enzyme triose phosphate isomerase rapidly inter-converts the molecules dihydroxyacetone phosphate and glyceraldehyde phosphate. Glyceraldehyde phosphate is removed as soon as it is formed to be used in the next step of glycolysis. Dihydroxyacetone phosphate (C3H5O3P1) → Glyceraldehyde phosphate (C3H5O3P1) Net result for steps 4 and 5: Fructose 1, 6-bisphosphate (C6H10O6P2) ↔ 2 molecules of Glyceraldehyde phosphate (C3H5O3P1) |
|
|
Glycolysis step 5
Which enzyme rapidly inter-converts the molecules dihydroxyacetone phosphate and glyceraldehyde phosphate. |
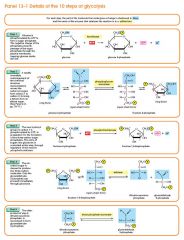
Glycolysis step 5
The enzyme triose phosphate isomerase rapidly inter-converts the molecules dihydroxyacetone phosphate and glyceraldehyde phosphate. Glyceraldehyde phosphate is removed as soon as it is formed to be used in the next step of glycolysis. Dihydroxyacetone phosphate (C3H5O3P1) → Glyceraldehyde phosphate (C3H5O3P1) Net result for steps 4 and 5: Fructose 1, 6-bisphosphate (C6H10O6P2) ↔ 2 molecules of Glyceraldehyde phosphate (C3H5O3P1) |
|
|
Glycolysis step 5
The enzyme triose phosphate isomerase rapidly inter-converts which molecules? |
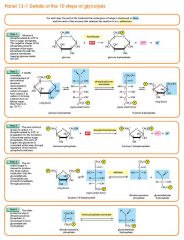
Glycolysis step 5
The enzyme triose phosphate isomerase rapidly inter-converts the molecules dihydroxyacetone phosphate and glyceraldehyde phosphate. Glyceraldehyde phosphate is removed as soon as it is formed to be used in the next step of glycolysis. Dihydroxyacetone phosphate (C3H5O3P1) → Glyceraldehyde phosphate (C3H5O3P1) Net result for steps 4 and 5: Fructose 1, 6-bisphosphate (C6H10O6P2) ↔ 2 molecules of Glyceraldehyde phosphate (C3H5O3P1) |
|
|
Where is glucokinase found in the body? |
Glucokinase (EC 2.7.1.2) is an enzyme that facilitates phosphorylation of glucose to glucose-6-phosphate. Glucokinase occurs in cells in the liver, pancreas, gut, and brain of humans and most other vertebrates. In each of these organs it plays an important role in the regulation of carbohydrate metabolism by acting as a glucose sensor, triggering shifts in metabolism or cell function in response to rising or falling levels of glucose, such as occur after a meal or when fasting. Mutations of the gene for this enzyme can cause unusual forms of diabetes or hypoglycemia.
Glucokinase (GK) is a hexokinase isozyme, related homologously and by evolution to at least three other hexokinases.[1] All of the hexokinases can mediate phosphorylation of glucose to glucose-6-phosphate (G6P), which is the first step of both glycogen synthesis and glycolysis. However, glucokinase is coded by a separate gene and its distinctive kinetic properties allow it to serve a different set of functions. Glucokinase has a lower affinity for glucose than the other hexokinases do, and its activity is localized to a few cell types, leaving the other three hexokinases as more important preparers of glucose for glycolysis and glycogen synthesis for most tissues and organs. Because of this reduced affinity, the activity of glucokinase, under usual physiological conditions, varies substantially according to the concentration of glucose. |
|
|
A hexokinase is an enzyme that does what?
|
A hexokinase is an enzyme that phosphorylates a six-carbon sugar, a hexose, to a hexose phosphate. In most tissues and organisms, glucose is the most important substrate of hexokinases, and glucose-6-phosphate the most important product.
|
|
|
What are the consequences of hexose phosphorylation?
|
The intracellular reactions mediated by hexokinases can be typified as:
Hexose-CH2OH + MgATP2− → Hexose-CH2O-PO2− 3 + MgADP− + H+ where hexose-CH2OH represents any of several hexoses (like glucose) that contain an accessible -CH2OH moiety. [edit] Consequences of hexose phosphorylationPhosphorylation of a hexose such as glucose often limits it to a number of intracellular metabolic processes, such as glycolysis or glycogen synthesis. Phosphorylation makes hexose unable to move or be transported out of the cell. |
|
|
Two important kinetic properties distinguish glucokinase from the other hexokinases?
|
Two important kinetic properties distinguish glucokinase from the other hexokinases, allowing it to function in a special role as glucose sensor.
Glucokinase has a lower affinity for glucose than the other hexokinases. Glucokinase changes conformation and/or function in parallel with rising glucose concentrations in the physiologically important range of 4-10 mmol/L (72-180 mg/dl). It is half-saturated at a glucose concentration of about 8 mmol/L (144 mg/dl).[6][7] Glucokinase is not inhibited by its product, glucose-6-phosphate.[6] This allows continued signal output (e.g., to trigger insulin release) amid significant amounts of its product |
|
|
Most of the glucokinase in a mammal is found where?
|
Most of the glucokinase in a mammal is found in the liver, and glucokinase provides approximately 95% of the hexokinase activity in hepatocytes. Phosphorylation of glucose to glucose-6-phosphate (G6P) by glucokinase is the first step of both glycogen synthesis and glycolysis in the liver.
|
|
|
Glucokinase provides approximately how much of the hexokinase activity in hepatocytes?
|
Most of the glucokinase in a mammal is found in the liver, and glucokinase provides approximately 95% of the hexokinase activity in hepatocytes. Phosphorylation of glucose to glucose-6-phosphate (G6P) by glucokinase is the first step of both glycogen synthesis and glycolysis in the liver.
|
|
|
As the glucose supply declines during periods of fasting, glucokinase activity in the cytoplasm diminishes. Which protein principally modulates GK activity and location by binding free cytoplasmic GK as glucose levels decline, and moving it into the nucleus, where it is held in reserve in an inactive form? |
Glucokinase (GK) in liver cells phosphorylates glucose, preparing it for incorporation into glycogen. During periods of ample glucose supply, most GK activity can be found in the peripheral cytoplasm where glycogen synthesis is occurring.[5] As the glucose supply declines during periods of fasting, GK activity in the cytoplasm diminishes. Glucokinase regulatory protein (GKRP) also known as glucokinase (hexokinase 4) regulator (GCKR) participates in this modulation of GK activity and location by binding free cytoplasmic GK as glucose levels decline, and moving it into the nucleus, where it is held in reserve in an inactive form. As glucose and insulin levels rise, as during digestion of a meal, GK is released from GKRP and moves back to the cytoplasm, where much of it associates with the bifunctional enzyme.
In hepatocytes of various mammals, GKRP has always been found in molar excess of the amount of GK, but the GKRP:GK ratio varies according to diet, insulin sufficiency, and other factors. Free GKRP shuttles between the nucleus and the cytoplasm. It may be attached to the microfilament cytoskeleton. GKRP competes with glucose to bind with GK, but inactivates it when bound. In conditions of low glucose, GKRP then pulls the GK into the nucleus. Rising amounts of glucose coming into the hepatocyte prompt the GKRP to rapidly release GK to return to the cytoplasm. GKRP itself is subject to modulation. Fructose and sorbitol can both be converted to fructose-1-phosphate, which inhibits GKRP and frees GK.[1] Fructose 6-phosphate binds to the same site of GKRP, but enhances the ability of GKRP to bind and inactivate GK. In contrast, phosphorylation of GKRP by AMP-activated protein kinase, induced by elevated levels of AMP, reduces its capacity to inactivate GK. |
|
|
GKRP itself is subject to modulation. Fructose and sorbitol can both be converted to what which inhibits GKRP and frees GK?
|
Glucokinase (GK) in liver cells phosphorylates glucose, preparing it for incorporation into glycogen. During periods of ample glucose supply, most GK activity can be found in the peripheral cytoplasm where glycogen synthesis is occurring.[5] As the glucose supply declines during periods of fasting, GK activity in the cytoplasm diminishes. GKRP participates in this modulation of GK activity and location by binding free cytoplasmic GK as glucose levels decline, and moving it into the nucleus, where it is held in reserve in an inactive form.[6] As glucose and insulin levels rise, as during digestion of a meal, GK is released from GKRP and moves back to the cytoplasm, where much of it associates with the bifunctional enzyme.[7]
GKRP competes with glucose to bind with GK, but inactivates it when bound. In conditions of low glucose, GKRP then pulls the GK into the nucleus. Rising amounts of glucose coming into the hepatocyte prompt the GKRP to rapidly release GK to return to the cytoplasm. GKRP itself is subject to modulation. Fructose and sorbitol can both be converted to fructose-1-phosphate, which inhibits GKRP and frees GK.[1] Fructose 6-phosphate binds to the same site of GKRP, but enhances the ability of GKRP to bind and inactivate GK. In contrast, phosphorylation of GKRP by AMP-activated protein kinase, induced by elevated levels of AMP, reduces its capacity to inactivate GK. |
|
|
Phosphofructokinase is a multisubunit protein. In such proteins, the subunits may behave cooperatively; that is, the activity of one subunit influences the activity of all the other subunits. Proteins that exhibit this behavior are known as what type of proteins? |
Phosphofructokinase is a multisubunit protein. In such proteins, the subunits may behave cooperatively; that is, the activity of one subunit influences the activity of all the other subunits. Proteins that exhibit this behavior are known as allosteric proteins. An especially notable example of an allosteric protein is hemoglobin, which is an oxygen-transport protein rather than an enzyme.
|
|
|
Phosphofructokinase (PFK) switches between two conformations, which are known as what?
|
PFK switches between two conformations, which are known as tense (T) and relaxed (R). When the enzyme is in the R state, it has a high affinity for its substrate, fructose-6-phosphate (F6P), and thus a high activity level. When PFK switches to the T state, its affinity for fructose-6-phosphate decreases, and consequently its activity level drops off. This switching between states is triggered by metabolites related to the the glycolytic pathway . An inhibitor such as PEP, an indicator of high energy in the cell, favors the T conformation of the enzyme. Fructose-6-phosphate binds poorly to PFK in the T state. On the other hand, an activator such as ADP favors the R state, to which fructose-6-phosphate binds better. How does the effector bound at the regulatory site influence the F6P binding site?
|
|
|
Many different effectors activate or inhibit the activity of Phosphofructokinase (PFK) . They all bind to the “effector” site of PFK. Two examples of such effectors are?
|
Since PFK catalyzes the committed step of glycolysis, it is regulated so that it will respond appropriately to the changing environmental and metabolic needs of the organism. Many different effectors activate or inhibit the activity of PFK. They all bind to the “effector” site of PFK. Two examples of such effectors are adenosine diphosphate (ADP) and PGA (a phosphoenolpyruvate analog). ADP activates PFK, and PGA inhibits PFK. It is clear that an inhibitor such as PEP favors the T conformation of the enzyme, to which fructose-6-phosphate binds poorly. Likewise, an activator such as ADP favors the R state, to which fructose-6-phosphate binds better. But how does the binding of an effector at a regulatory site influence the binding of fructose-6-phosphate at a relatively distant site on the enzyme? A model of how this activation/inhibition occurs is presented here.
|
|
|
PFK1 is allosterically inhibited by high levels of what?
|
PFK1 is the most important control site in the mammalian glycolytic pathway. This step is subject to extensive regulation since it is not only highly endergonic under physiological conditions, but also because it is the committed step - the first irreversible reaction unique to the glycolytic pathway. This leads to a precise control of glucose and the other monosaccharides galactose and fructose going down the glycolysis pathway. Before this enzyme's reaction, glucose-6-phosphate can potentially travel down the pentose phosphate pathway, or be converted to glucose-1-phosphate for glycogenesis
PFK1 is allosterically inhibited by high levels of ATP but AMP reverses the inhibitory action of ATP. Therefore, the activity of the enzyme increases when the cellular ATP/AMP ratio is lowered. Glycolysis is thus stimulated when energy charge falls. PFK1 has two sites with different affinities for ATP which is both a substrate and an inhibitor.[1] PFK1 is also inhibited by low pH levels which augment the inhibitory effect of ATP. The pH falls when muscle is functioning anaerobically and producing excessive quantities of lactic acid. This inhibitory effect serves to protect the muscle from damage that would result from the accumulation of too much acid.[1] Finally, PFK1 is allosterically inhibited by both PEP and citrate. Phosphoenolpyruvic acid is a product further downstream the glycolytic pathway and citrate is an early intermediate in the citric acid cycle. Citrate buildup is a sign of the citric acid cycle reaching saturation and thus glycolysis slows down as there is no longer any need to commit more glucose to degradation. PFK1 is allosterically activated by a high concentration of AMP, but the most potent activator is fructose 2,6-bisphosphate, which is also produced from fructose-6-phosphate by PFK2. Hence, an abundance of F6P results in a higher concentration of fructose 2,6-bisphosphate (F-2,6-BP). The binding of F-2,6-BP increases the affinity of PFK1 for F6P and diminishes the inhibitory effect of ATP. This is an example of feedforward stimulation as glycolysis is accelerated when glucose is abundant.[1] PFK is inhibited by glucagon through repression of synthesis. Glucagon activates protein kinase A which, in turn, shuts off the kinase activity of PFK2. This reverses any synthesis of F-2,6-BP from F6P and thus inhibits PFK1 activity. The precise regulation of PFK1 prevents glycolysis and gluconeogenesis from occurring simultaneously. However, there is substrate cycling between F6P and F-1,6-BP. Fructose-1,6-bisphosphatase (FBPase) catalyzes the hydrolysis of F-1,6-BP back to F6P, the reverse reaction catalyzed by PFK1. There is a small amount of FBPase activity during glycolysis and some PFK1 activity during gluconeogenesis. This cycle allows for the amplification of metabolic signals as wells as the generation of heat by ATP hydrolysis. |
|
|
PFK1 is allosterically inhibited by high levels of ATP but what reverses the inhibitory action of ATP?
|
PFK1 is the most important control site in the mammalian glycolytic pathway. This step is subject to extensive regulation since it is not only highly endergonic under physiological conditions, but also because it is the committed step - the first irreversible reaction unique to the glycolytic pathway. This leads to a precise control of glucose and the other monosaccharides galactose and fructose going down the glycolysis pathway. Before this enzyme's reaction, glucose-6-phosphate can potentially travel down the pentose phosphate pathway, or be converted to glucose-1-phosphate for glycogenesis
PFK1 is allosterically inhibited by high levels of ATP but AMP reverses the inhibitory action of ATP. Therefore, the activity of the enzyme increases when the cellular ATP/AMP ratio is lowered. Glycolysis is thus stimulated when energy charge falls. PFK1 has two sites with different affinities for ATP which is both a substrate and an inhibitor.[1] PFK1 is also inhibited by low pH levels which augment the inhibitory effect of ATP. The pH falls when muscle is functioning anaerobically and producing excessive quantities of lactic acid. This inhibitory effect serves to protect the muscle from damage that would result from the accumulation of too much acid.[1] Finally, PFK1 is allosterically inhibited by both PEP and citrate. Phosphoenolpyruvic acid is a product further downstream the glycolytic pathway and citrate is an early intermediate in the citric acid cycle. Citrate buildup is a sign of the citric acid cycle reaching saturation and thus glycolysis slows down as there is no longer any need to commit more glucose to degradation. PFK1 is allosterically activated by a high concentration of AMP, but the most potent activator is fructose 2,6-bisphosphate, which is also produced from fructose-6-phosphate by PFK2. Hence, an abundance of F6P results in a higher concentration of fructose 2,6-bisphosphate (F-2,6-BP). The binding of F-2,6-BP increases the affinity of PFK1 for F6P and diminishes the inhibitory effect of ATP. This is an example of feedforward stimulation as glycolysis is accelerated when glucose is abundant.[1] PFK is inhibited by glucagon through repression of synthesis. Glucagon activates protein kinase A which, in turn, shuts off the kinase activity of PFK2. This reverses any synthesis of F-2,6-BP from F6P and thus inhibits PFK1 activity. The precise regulation of PFK1 prevents glycolysis and gluconeogenesis from occurring simultaneously. However, there is substrate cycling between F6P and F-1,6-BP. Fructose-1,6-bisphosphatase (FBPase) catalyzes the hydrolysis of F-1,6-BP back to F6P, the reverse reaction catalyzed by PFK1. There is a small amount of FBPase activity during glycolysis and some PFK1 activity during gluconeogenesis. This cycle allows for the amplification of metabolic signals as wells as the generation of heat by ATP hydrolysis. |
|
|
Phosphofructosekinase is allosterically activated by a high concentration of AMP, but the most potent activator is?
|
PFK1 is allosterically activated by a high concentration of AMP, but the most potent activator is fructose 2,6-bisphosphate, which is also produced from fructose-6-phosphate by PFK2. Hence, an abundance of F6P results in a higher concentration of fructose 2,6-bisphosphate (F-2,6-BP). The binding of F-2,6-BP increases the affinity of PFK1 for F6P and diminishes the inhibitory effect of ATP. This is an example of feedforward stimulation as glycolysis is accelerated when glucose is abundant
|
|
|
Glycolysis step 6
Which enzyme serves two functions in this step? |
Glycolysis step 6
The enzyme triose phosphate dehydrogenase serves two functions in this step. First the enzyme transfers a hydrogen (H-) from glyceraldehyde phosphate to the oxidizing agent nicotinamide adenine dinucleotide (NAD+) to form NADH. Next triose phosphate dehydrogenase adds a phosphate (P) from the cytosol to the oxidized glyceraldehyde phosphate to form 1, 3-bisphosphoglycerate. This occurs for both molecules of glyceraldehyde phosphate produced in step 5. A. Triose phosphate dehydrogenase + 2 H- + 2 NAD+ → 2 NADH + 2 H+ B. Triose phosphate dehydrogenase + 2 P + 2 glyceraldehyde phosphate (C3H5O3P1) → 2 molecules of 1,3-bisphosphoglycerate (C3H4O4P2) |
|
|
Glycolysis step 6
The enzyme triose phosphate dehydrogenase serves two functions in this step. First the enzyme transfers a hydrogen (H-) from which oxidizing agent to form what? |
Glycolysis step 6
The enzyme triose phosphate dehydrogenase serves two functions in this step. First the enzyme transfers a hydrogen (H-) from glyceraldehyde phosphate to the oxidizing agent nicotinamide adenine dinucleotide (NAD+) to form NADH. Next triose phosphate dehydrogenase adds a phosphate (P) from the cytosol to the oxidized glyceraldehyde phosphate to form 1, 3-bisphosphoglycerate. This occurs for both molecules of glyceraldehyde phosphate produced in step 5. A. Triose phosphate dehydrogenase + 2 H- + 2 NAD+ → 2 NADH + 2 H+ B. Triose phosphate dehydrogenase + 2 P + 2 glyceraldehyde phosphate (C3H5O3P1) → 2 molecules of 1,3-bisphosphoglycerate (C3H4O4P2) |
|
|
Glycolysis step 6
The enzyme triose phosphate dehydrogenase serves two functions in this step. First the enzyme transfers a hydrogen (H-) from glyceraldehyde phosphate to the oxidizing agent nicotinamide adenine dinucleotide (NAD+) to form NADH. Next triose phosphate dehydrogenase adds what from the cytosol to the oxidized what to form 1, 3-bisphosphoglycerate? |
Glycolysis step 6
The enzyme triose phosphate dehydrogenase serves two functions in this step. First the enzyme transfers a hydrogen (H-) from glyceraldehyde phosphate to the oxidizing agent nicotinamide adenine dinucleotide (NAD+) to form NADH. Next triose phosphate dehydrogenase adds a phosphate (P) from the cytosol to the oxidized glyceraldehyde phosphate to form 1, 3-bisphosphoglycerate. This occurs for both molecules of glyceraldehyde phosphate produced in step 5. A. Triose phosphate dehydrogenase + 2 H- + 2 NAD+ → 2 NADH + 2 H+ B. Triose phosphate dehydrogenase + 2 P + 2 glyceraldehyde phosphate (C3H5O3P1) → 2 molecules of 1,3-bisphosphoglycerate (C3H4O4P2) |
|
|
Describe glycolysis step 7
|
Glycolysis step 7
The enzyme phosphoglycerokinase transfers a P from 1,3-bisphosphoglycerate to a molecule of ADP to form ATP. This happens for each molecule of 1,3-bisphosphoglycerate. The process yields two 3-phosphoglycerate molecules and two ATP molecules. 2 molecules of 1,3-bisphoshoglycerate (C3H4O4P2) + phosphoglycerokinase + 2 ADP → 2 molecules of 3-phosphoglycerate (C3H5O4P1) + 2 ATP |
|
|
Glycolysis step 7
Which enzyme transfers a P from 1,3-bisphosphoglycerate to a molecule of ADP to form ATP? |
Glycolysis step 7
The enzyme phosphoglycerokinase transfers a P from 1,3-bisphosphoglycerate to a molecule of ADP to form ATP. This happens for each molecule of 1,3-bisphosphoglycerate. The process yields two 3-phosphoglycerate molecules and two ATP molecules. 2 molecules of 1,3-bisphoshoglycerate (C3H4O4P2) + phosphoglycerokinase + 2 ADP → 2 molecules of 3-phosphoglycerate (C3H5O4P1) + 2 ATP |
|
|
Glycolysis step 7
The enzyme phosphoglycerokinase transfers a P from what to what to form ATP? |
Glycolysis step 7
The enzyme phosphoglycerokinase transfers a P from 1,3-bisphosphoglycerate to a molecule of ADP to form ATP. This happens for each molecule of 1,3-bisphosphoglycerate. The process yields two 3-phosphoglycerate molecules and two ATP molecules. 2 molecules of 1,3-bisphoshoglycerate (C3H4O4P2) + phosphoglycerokinase + 2 ADP → 2 molecules of 3-phosphoglycerate (C3H5O4P1) + 2 ATP |
|
|
Describe glycolysis step 8 |
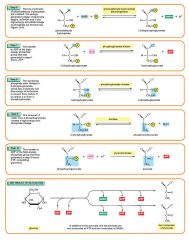
Step 8
The enzyme phosphoglyceromutase relocates the P from 3-phosphoglycerate from the third carbon to the second carbon to form 2-phosphoglycerate. 2 molecules of 3-Phosphoglycerate (C3H5O4P1) + phosphoglyceromutase → 2 molecules of 2-Phosphoglycerate (C3H5O4P1) |
|
|
Glycolysis step 8
which enzyme relocates the P from 3-phosphoglycerate from the third carbon to the second carbon to form 2-phosphoglycerate? |
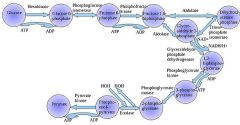
Step 8
The enzyme phosphoglyceromutase relocates the P from 3-phosphoglycerate from the third carbon to the second carbon to form 2-phosphoglycerate. 2 molecules of 3-Phosphoglycerate (C3H5O4P1) + phosphoglyceromutase → 2 molecules of 2-Phosphoglycerate (C3H5O4P1) |
|
|
Glycolysis step 8
The enzyme phosphoglyceromutase relocates the P from the third carbon to the second carbon of what to form what? |
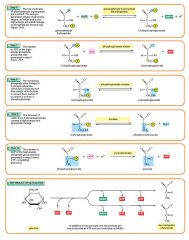
Step 8
The enzyme phosphoglyceromutase relocates the P from 3-phosphoglycerate from the third carbon to the second carbon to form 2-phosphoglycerate. 2 molecules of 3-Phosphoglycerate (C3H5O4P1) + phosphoglyceromutase → 2 molecules of 2-Phosphoglycerate (C3H5O4P1) |
|
|
Glycolysis step 9
Which enzyme removes a molecule of water from 2-phosphoglycerate to form phosphoenolpyruvic acid (PEP)? |
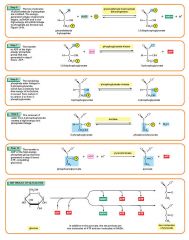
Glycolysis step 9
The enzyme enolase removes a molecule of water from 2-phosphoglycerate to form phosphoenolpyruvic acid (PEP). This happens for each molecule of 2-phosphoglycerate. 2 molecules of 2-Phosphoglycerate (C3H5O4P1) + enolase → 2 molecules of phosphoenolpyruvic acid (PEP) (C3H3O3P1) |
|
|
Glycolysis step 9
The enzyme enolase removes a molecule of water from what to form what? |
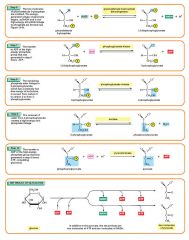
Glycolysis step 9
The enzyme enolase removes a molecule of water from 2-phosphoglycerate to form phosphoenolpyruvic acid (PEP). This happens for each molecule of 2-phosphoglycerate. 2 molecules of 2-Phosphoglycerate (C3H5O4P1) + enolase → 2 molecules of phosphoenolpyruvic acid (PEP) (C3H3O3P1) |
|
|
Glycolysis step 10
The enzyme pyruvate kinase transfers a P from what to what to form pyruvic acid and ATP? |
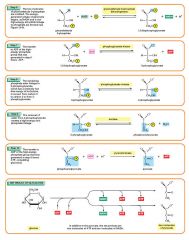
Glycolysis step 10
The enzyme pyruvate kinase transfers a P from PEP to ADP to form pyruvic acid and ATP. This happens for each molecule of PEP. This reaction yields 2 molecules of pyruvic acid and 2 ATP molecules. 2 molecules of PEP (C3H3O3P1) + pyruvate kinase + 2 ADP → 2 molecules of pyruvic acid (C3H4O3) + 2 ATP |
|
|
Glycolysis step 10
The enzyme pyruvate kinase transfers a P from PEP to ADP to form what as well as ATP? This reaction yields how many molecules of ATP? |
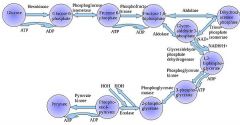
Glycolysis step 10
The enzyme pyruvate kinase transfers a P from PEP to ADP to form pyruvic acid and ATP. This happens for each molecule of PEP. This reaction yields 2 molecules of pyruvic acid and 2 ATP molecules. 2 molecules of PEP (C3H3O3P1) + pyruvate kinase + 2 ADP → 2 molecules of pyruvic acid (C3H4O3) + 2 ATP |
|
|
Pathophysiology of arsenic poisoning?
|
Pathophysiology
Inorganic forms of arsenic are more toxic than organic forms. The trivalent forms are more toxic and react with thiol groups, while the pentavalent forms are less toxic but uncouple oxidative phosphorylation. Very few organ systems escape the toxic effects of arsenic. Trivalent inorganic arsenic inhibits pyruvate dehydrogenase by binding to the sulfhydryl groups of dihydrolipoamide. Consequently, conversion of pyruvate to acetyl coenzyme A (CoA) is decreased, citric acid cycle activity is decreased, and production of cellular ATP is decreased. Trivalent arsenic inhibits numerous other cellular enzymes through sulfhydryl group binding. Trivalent arsenic inhibits cellular glucose uptake, gluconeogenesis, fatty acid oxidation, and further production of acetyl CoA; it also blocks the production of glutathione, which prevents cellular oxidative damage. Effects of pentavalent inorganic arsenic occur partially because of its transformation to trivalent arsenic; toxicity proceeds as outlined above. More importantly, pentavalent arsenic resembles inorganic phosphate and substitutes for phosphate in glycolytic and cellular respiration pathways. Consequently, high-energy phosphate bonds are not made, and uncoupling of oxidative phosphorylation occurs. For example, in the presence of pentavalent arsenic, adenosine diphosphate (ADP) forms ADP-arsenate instead of ATP; the high-energy phosphate bonds of ATP are lost. |
|
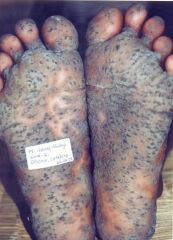
Diagnosis?
|
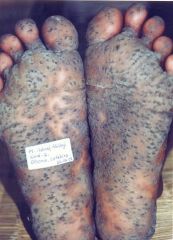
Blackfoot disease (BFD) is a severe form of peripheral vascular disease (PVD), in which the blood vessels in the lower limbs are severely damaged, resulting eventually in progressive gangrene.
Blackfoot Disease (BFD), a peripheral vascular disease associated with long-term arsenic exposure on the southwest coast of Taiwan. It is characterized by severe systemic arteriosclerosis as well as dry gangrene and spontaneous amputations of affected extremities and end stages.” Dr. Chien-Jen Chen stated, “In addition to Blackfoot Disease, arsenic in drinking water may lead to the development of several cancers in humans. Studies have shown a significant dose-response relationship between arsenic in drinking water and increased risk of cancers of the liver, nasal cavity, lung, skin, urinary bladder, kidney and prostate in southwestern and northeastern Taiwan. Sufficient evidences in humans have also revealed that arsenic in drinking water has caused cancers of urinary bladder, lung and skin. |
|
|
Patients with acute arsenic exposure usually develop symptoms within 30 minutes. They present with?
|
Patients with acute arsenic exposure usually develop symptoms within 30 minutes. They present with? gastrointestinal distress characterized by nausea, vomiting, abdominal pain, and profuse watery or bloody diarrhea. Patients often are hypotensive and tachycardic and may complain of a metallic taste in their mouth and have a garlic odor on their breath.1 Patients frequently exhibit signs of delirium upon examination.
|
|
|
Patients with chronic arsenic exposure often present with the complaint of painful paresthesias. Neuropathy results in?
|
Patients with chronic arsenic exposure often present with the complaint of painful paresthesias. Neuropathy results in diminished sensitivity to pinprick, light touch, temperature, and vibration and in motor deficits in a stocking-glove distribution. Muscle wasting and foot drop sometimes are noted. Other examination findings include cyanosis of distal extremities, pallor from anemia, hyperpigmentation of skin, and Mees lines. Patients may develop cardiovascular effects, diabetes mellitus, or cancer as well.
Patients with acute arsine gas exposure present with headache, nausea, vomiting, diarrhea, and abdominal pain. Patients often develop dyspnea and severe jaundice. |
|
|
2,3-BPG (DPG) is formed from?
|
2,3-BPG is formed from 1,3-BPG by the enzyme 2,3-BPG mutase. It can then be broken down by 2,3-BPG phosphatase to form 3-phosphoglycerate. Its synthesis and breakdown are, therefore, a way around a step of glycolysis.
|
|
|
Erythrocytes synthesize and degrade the 2.3-BPG by a diversion of what?
|
Erythrocytes synthesize and degrade the 2.3-BPG by a diversion of the glycolytic pathway.
The first phase of glucose catabolism includes glucose phosphorylation, isomerization and another phosphorylation to bear fructose-1,6-bisphosphate (F-1,6-BP). Cleavage of fructose 1, 6-bisphosphate yields two molecules: glyceraldehyde-3-phosphate (G3P) and dihydroxyacetone phosphate (DHAP). These two molecules are isomers and are readily converted into one another by triose-phosphate isomerase. The equilibrium of this conversion lies heavily on the side of DHAP for two reasons. Firstly so the glycolytic pathway does not get oversaturated and secondly so that the biochemistry of glycerol can be tied into glycolysis. DHAP can be converted into glycerol when the supply of DHAP is plentiful and glycerol can then be used as a substrate for phase 2 glycolysis when glycolytic intermediates are scarce. The second phase of glucose catabolism converts G3P to 3-phosphoglycerate (3-PG). During the first reaction step, G3P is phosphorylated with a high-energy phosphate and oxidized to 1,3-bisphosphoglycerate (1,3-BPG), through the action of glyceraldehyde 3-phosphate dehydrogenase (G3PD). 1,3-BPG may be dephosphorylated by phosphoglycerate kinase (PGK), generating ATP, or it may be shunted into the Luebering-Rapapport pathway, where bisphosphoglycerate mutase catalyzes the transfer of a phosphoryl group from C1 to C2 of 1,3-BPG, giving 2,3-BPG. 2,3-BPG, the most concentrated organophosphate in the erythrocyte, forms 3-PG by the action of bisphosphoglycerate phosphatase. The concentration on 2,3-BPG varies inversely with the pH, which is inhibitory to catalytic action of bisphosphoglyceromutase. The third phase of anaerobic glucose catabolism involves conversion of 3-PG to pyruvate with the generation of ATP. There is a delicate balance between the need to generate ATP to support energy requirements for cell metabolism and the need to maintain appropriate oxygenation/deoxygenation status of hemoglobin. This balance is maintained by dephosphorilation of 1,3-BPG to 2,3-BPG, which enhances the deoxygenation of hemoglobin. Low pH inhibits the activity of biphosphoglyceromutase and activates bisphosphoglyerate phosphatase, which favors generation of ATP |
|
|
2,3-Bisphosphoglyceric acid (2,3-Bisphosphoglycerate or 2,3-BPG, also known as 2,3-diphosphoglycerate or 2,3-DPG) interacts with which deoxygenated hemoglobin subunits? |
2,3-Bisphosphoglyceric acid (2,3-Bisphosphoglycerate or 2,3-BPG, also known as 2,3-diphosphoglycerate or 2,3-DPG) is a three-carbon isomer of the glycolytic intermediate 1,3-bisphosphoglyceric acid (1,3-BPG). 2,3-BPG is present in human red blood cells (RBC; erythrocyte) at approximately 5 mmol/L. It binds with greater affinity to deoxygenated hemoglobin (e.g. when the red cell is near respiring tissue) than it does to oxygenated hemoglobin (e.g., in the lungs) due to spatial changes: 2,3-BPG (whose size is estimated at about 9 angstroms) fits in the deoxygenated hemoglobin configuration (11 angstroms), but not as well in the oxygenated (5 angstroms). It interacts with deoxygenated hemoglobin beta subunits by decreasing their affinity for oxygen, so it allosterically promotes the release of the remaining oxygen molecules bound to the hemoglobin, thus enhancing the ability of RBCs to release oxygen near tissues that need it most. 2,3-BPG is thus an allosteric effector.
|
|
|
When 2,3-BPG binds to deoxyhemoglobin, it acts to stabilize what?
|
When 2,3-BPG binds to deoxyhemoglobin, it acts to stabilize the low oxygen affinity state (T state) of the oxygen carrier. It fits neatly into the cavity of the deoxy- conformation, exploiting the molecular symmetry and positive polarity by forming salt bridges with lysine and histidine residues in the four subunits of hemoglobin. The R state, with oxygen bound to a heme group, has a different conformation and does not allow this interaction. By itself, hemoglobin has sigmoid-like kinetics, which makes easier another subunits’ binding (the first molecule of oxygen helps the following to link).
By selectively binding to deoxyhemoglobin, 2,3-BPG stabilizes the T state conformation, making it harder for oxygen to bind hemoglobin and more likely to be released to adjacent tissues. 2,3-BPG is part of a feedback loop that can help prevent tissue hypoxia in conditions where it is most likely to occur. Conditions of low tissue oxygen concentration such as high altitude (2,3-BPG levels are higher in those acclimated to high altitudes), airway obstruction, or congestive heart failure will tend to cause RBCs to generate more 2,3-BPG in their effort to generate energy by allowing more oxygen to be released in tissues deprived of oxygen. Ultimately, this mechanism increases oxygen release from RBCs under circumstances where it is needed most. This release is potentiated by the Bohr effect in tissues with high energetic demands. Bohr effect is another useful way to solve the affinity problem of the hemoglobin, and it’s related to the pH and the CO2. It’s important to highlight that the behaviour of myoglobin doesn’t work in the same way, as 2,3-BPG has no effect on it. |
|
|
Fetal hemoglobin (HbF) exhibits a low affinity for 2,3-BPG, resulting in what?
|
Fetal hemoglobin (HbF) exhibits a low affinity for 2,3-BPG, resulting in a higher binding affinity for oxygen. This increased oxygen-binding affinity relative to that of adult hemoglobin (HbA) is due to HbF's having two α/γ dimers as opposed to the two α/β dimers of HbA. The positive histidine residues of HbA β-subunits that are essential for forming the 2,3-BPG binding pocket are replaced by serine residues in HbF γ-subunits. Like that, histidine nº143 gets lost, so 2,3-BPG has difficulties in linking to the fetal hemoglobin, and it looks like the pure hemoglobin. That’s the way O2 flows from the mother to the fetus. As we can see in the following image, fetal hemoglobin has more affinity to oxygen than adult hemoglobin. Moreover, myoglobin has the highest affinity to oxygen.
|
|
|
What is substrate-level phosphorylation?
|
Substrate-level phosphorylation is a type of metabolism that results in the formation and creation of adenosine triphosphate (ATP) or guanosine triphosphate (GTP) by the direct transfer and donation of a phosphoryl (PO3) group to adenosine diphosphate (ADP) or guanosine diphosphate (GDP) from a phosphorylated reactive intermediate. By convention, the phosphoryl group that is transferred is referred to as a phosphate group.
An alternative way to create ATP is through oxidative phosphorylation, which takes place during the process of cellular respiration, in addition to the substrate-level phosphorylation that occurs during glycolysis and the Krebs cycle. During oxidative phosphorylation, NADH is oxidized to NAD+, yielding 2.5 ATPs, and FADH2 yields 1.5 ATPs when it is oxidized. Oxidative phosphorylation uses electrochemical or chemiosmotic gradient of protons (H+) across the inner mitochondrial membrane to generate ATP from ADP, which is a key difference from substrate-level phosphorylation. Unlike oxidative phosphorylation, oxidation and phosphorylation are not joined in the process of Substrate-level phosphorylation, although both types of phosphorylation result in ATP and reactive intermediates are most often gained in course of oxidation processes in catabolism. However, usually most of the ATP is generated by oxidative phosphorylation in aerobic or anaerobic respiration. Substrate-level phosphorylation serves as fast source of ATP independent of external electron acceptors and respiration. This is the case for example in human erythrocytes, which have no mitochondria, and in the muscle during oxygen depression. The main part of Substrate-level phosphorylation occurs in the cytoplasm of cells as part of glycolysis and in mitochondria as part of the Krebs Cycle under both aerobic and anaerobic conditions. |
|
|
Glycolysis, Step 10?
|
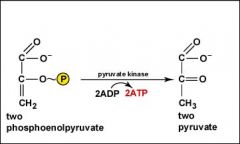
As the two molecules of phosphoenolpyruvate are converted to two molecules of pyruvate, the high-energy phosphate groups are added to ADP producing 2 ATP by substrate-level phosphorylation.
|
|
|
Glycolysis, Step 3?
|
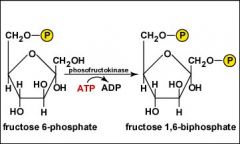
A second molecule of ATP is hydrolyzed to transfer a phosphate group to the number 1 carbon of fructose 6-phosphate to produce fructose 1,6-biphosphate.
|
|
|
Glycolysis, Step 4?
|
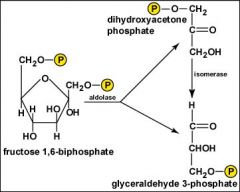
The 6-carbon fructose 1,6 biphosphate is split to form two, 3-carbon molecules: glyceraldehyde 3-phosphate and dihydroxyacetone phosphate. The dihydroxyacetone phosphate is then converted into a second molecule of glyceraldehyde 3-phosphate. Two molecules of glyceraldehyde 3-phosphate will now go through each of the remaining steps in glycolysis producing two molecules of each product.
|
|
|
Glycolysis, Step 6?
|
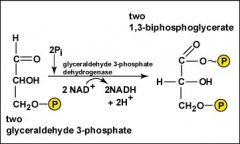
As each of the two molecules of glyceraldehyde 3-phosphate are oxidized, the energy released is used to add an inorganic phosphate group to form two molecules of 1,3-biphosphoglycerate, each containing a high-energy phosphate bond. During these oxidations, two molecules of NAD+ are reduced to form two NADH + 2H+.
|
|
|
Glycolysis, Step 7?
|
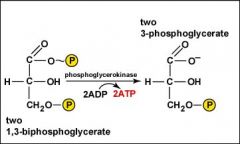
As each of the two molecules of 1,3-biphosphoglycerate are converted to 3-phosphoglycerate, the high-energy phosphate group is added to ADP producing 2 ATP by substrate-level phosphorylation.
|
|
|
Glycolysis, Step 8?
|
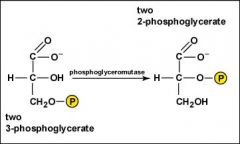
The two molecules of 3-phosphoglycerate are rearranged to form two molecules of 2-phosphoglycerate.
|
|
|
Glycolysis, Step 9?
|
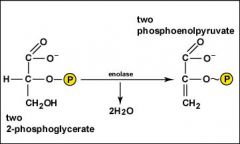
Water is removed from each of the two molecules of 2-phosphoglycerate converting the phosphate bonds to a high-energy phosphate bonds as two molecules of phosphoenolpyruvate are produced.
|
|
|
Pyruvate kinase is an enzyme involved in glycolysis. It catalyzes the transfer what from phosphoenolpyruvate (PEP) to what?
|
Pyruvate kinase (EC: 2.7.1.40) is an enzyme involved in glycolysis. It catalyzes the transfer of a phosphate group from phosphoenolpyruvate (PEP) to ADP, yielding one molecule of pyruvate and one molecule of ATP.
|
|
|
Pyruvate kinase activity is regulated by?
|
Pyruvate kinase activity is regulated by
Its own substrate PEP and fructose 1,6-bisphosphate, an intermediate in glycolysis, both of which enhance enzymatic activity. Thus, glycolysis is driven to operate faster when more substrate is present. ATP is a negative allosteric inhibitor. This accounts for parallel regulation with PFK 1. It is not known whether citrate plays a role in negative allosteric inhibition, however it is believed that acetyl-CoA does. Alanine, a negative allosteric modulator Liver pyruvate kinase is also regulated indirectly by epinephrine and glucagon, through protein kinase A. This protein kinase phosphorylates liver pyruvate kinase to deactivate it. Muscle pyruvate kinase is not inhibited by epinephrine activation of protein kinase A. Glucagon signals fasting (no glucose available). Thus, glycolysis is inhibited in the liver but unaffected in muscle when fasting. An increase in blood sugar leads to secretion of insulin, which activates phosphoprotein phosphatase I, leading to dephosphorylation and activation of pyruvate kinase. These controls prevent pyruvate kinase from being active at the same time as the enzymes that catalyze the reverse reaction (pyruvate carboxylase and phosphoenolpyruvate carboxykinase), preventing a futile cycle. |
|
|
Pyruvate kinase deficiency. In this condition, a lack of pyruvate kinase slows down the process of glycolysis. This effect is especially devastating in cells that lack what?
|
Genetic defects of this enzyme cause the disease known as pyruvate kinase deficiency. In this condition, a lack of pyruvate kinase slows down the process of glycolysis. This effect is especially devastating in cells that lack mitochondria, because these cells must use anaerobic glycolysis as their sole source of energy because the TCA cycle is not available.
One example is red blood cells, which in a state of pyruvate kinase deficiency rapidly become deficient in ATP and can undergo hemolysis. Therefore, pyruvate kinase deficiency can cause hemolytic anemia. |
|
|
Pyruvate kinase also serves as a regulatory enzyme for gluconeogenesis, a biochemical pathway in which the liver generates glucose from pyruvate and other substrates. When pyruvate kinase is inhibited by phosphorylation (which occurs in the fasting state, via glucagon), what is prevented from being converted to what?
|
Pyruvate kinase also serves as a regulatory enzyme for gluconeogenesis, a biochemical pathway in which the liver generates glucose from pyruvate and other substrates. When pyruvate kinase is inhibited by phosphorylation (which occurs in the fasting state, via glucagon), phosphoenolpyruvate is prevented from being converted to pyruvate. Instead, it is converted to glucose in a series of gluconeogenesis reactions that are mostly (but not exactly) the reverse sequence of glycolysis.
The glucose thus produced is expelled from the liver, providing energy for vital tissues in the fasting state. |
|
|
final step of glycolysis is formation of pyruvate. At this step, pyruvate forms either what or enters what? |
final step of glycolysis is formation of pyruvate. At this step, pyruvate forms either into lactate (lactate dehydrogenase, LDH) or enters the mitochondria and the Kreb's cycle
|
|
|
Normally, NAD+ is reduced to NADH that deposit the H+ at the electron transport gate (ETC) in the mitrochondria to be combined with oxygen to form water (H2O).
If there is insufficient oxygen then NADH cannot release the H+ and they build up in the cell. To prevent the rise in acidity, what accepts the H+ to form what? |
Normally, NAD+ is reduced to NADH that deposit the H+ at the electron transport gate (ETC) in the mitrochondria to be combined with oxygen to form water (H2O).
If there is insufficient oxygen then NADH cannot release the H+ and they build up in the cell. To prevent the rise in acidity pyruvic acid accepts H+ forming lactic acid that then dissociates into lactate and H+. Some of the lactate diffuses into the blood stream and takes some H+ with it as a way of reducing the H+ concentration in the muscle cell. The normal pH of the muscle cell is 7.1 but if the build up of H+ continues and pH is reduced to around 6.5 then muscle contraction may be impaired and the low pH will stimulate the free nerve endings in the muscle resulting in the perception of pain (the burn). This point is often measured as the lactic threshold or anaerobic threshold (AT) or onset of blood lactate accumulation (OBLA). |
|
|
One step conversion of Pyruvate to Lactate is catalysed by?
|
One step conversion of Pyruvate to Lactate is catalysed by Lactate dehydrogenase.
|
|
|
5. One of the differences between aerobic and anaerobic respiration is that in anaerobic respiration
(1.) glucose is utilized in energy production (2.) ethyl alcohol may be a product (3.) ATP is a product (4.) carbon dioxide is a product (5.) NAD is one of the coenzymes |
5. 2
|
|
|
6. Muscle fatigue results from the accumulation of lactic acid in the muscles because of
(1.) enzyme deficiency (2.) oxygen deficiency (3.) excess number of mitochondria in the cell (4.) too much oxygen in the cells (5.) Inability to metabolize glucose |
6. 2
|
|
|
9. Glycolysis without oxygen (1.) requires pyruvate (2.) produces alcohol or lactic acid
(3.) occurs mainly in the mitochondria (4.) requires no activation energy |
9. 2
|
|
|
10. The initial end product of anaerobic glycolysis.
(1.) carbon dioxide (2.) pyruvate (3.) ethyl alcohol (4.) acetyl CoA |
10. 2
|
|
|
11. One end product of aerobic glycolysis. (2 possible answers here -- don't panic!)
(1.) carbon dioxide (2.) pyruvate (3.) ethyl alcohol (4.) acetyl CoA |
11. 4
|
|
|
12. Two of these are released in one turn of the Kreb's Cycle.
(1.) carbon dioxide (2.) pyruvate (3.) ethyl alcohol (4.) acetyl CoA |
12. 1
|
|
|
13. The end product of fermentation other than ATP or carbon dioxide.
(1.) carbon dioxide (2.) pyruvate (3.) ethyl alcohol (4.) acetyl CoA |
13. 3
|
|
|
14. Which is NOT an end product of the common pathway of glycolysis?
(1.) pyruvate (2.) ATP (3.) reduced NAD (4.) glucose |
14. 4
|
|
|
15. ATP is used in a cell to (1.) accept energy (2.) become part of the nuclear membranes
(3.) regulate membrane permeability (4.) store energy (5.) digest polysaccharides |
Answers
15. 4 |
|
|
16. Where does anaerobic glycolysis occur in living things? (1.) golgi complex (2.) cytoplasm
(3.) mitochondria (4.) endoplasmic reticulum |
16. 2
|
|
|
17. The primary role of oxygen in respiration is to
(1.) yield energy in the form of ATP as it is passed down the respiratory chain (2.) act as an acceptor for electrons and hydrogen, forming water (3.) combine with carbon forming carbon dioxide (4.) combine with lactic acid to from pyruvic acid (5.) catalyze glycolysis reactions |
17. 2
|
|
|
18. Oxidative phosphorylation can be carried out in a test tube in the absence of intact cells,
provided certain subcellular material is used. Assuming that the test tube contains all of the necessary substrates, what subcellular material would you expect to be the minimum requirement for the process to proceed? (1.) intact outer mitochondrial membranes (2.) vesicles composed of inner mitochondrial membrane (3.) intact thylakoid membranes (4.) golgi complex fractions |
18. 1
|
|
|
19. For each glucose entering respiration, the citric acid cycle itself produces
(1.) eight carbon dioxide molecules (2.) two carbon dioxide molecules (3.) three carbon dioxide molecules (4.) four carbon dioxide molecules |
19. 4
|
|
|
20. Which intermediary metabolites enters the Krebs cycle and is formed, in part,
by the removal of carbon dioxide from a molecule of pyruvate? (1.) lactic acid (2.) phosphoglyceraldehyde (3.) oxaloacetic acid (4.) acetyl CoA (5.) citric acid |
20. 4
|
|
|
21. When hydrogen ions are pumped out of the mitochondrial matrix,
across the inner mitochondrial membrane, and into the outer compartment, the result is (1.) damage to the mitochondrion (2.) the restoration of NAD (3.) the restoration of the Na-K balance across the membrane (4.) the creation of a proton gradient (5.) the lowering of pH in the mitochondrial matrix |
21. 4
|
|
|
22. Which statement about anaerobic respiration is FALSE:
(1.) The products are at a higher free energy level than the reactants. (2.) It is sufficient to satisfy the energy requirements of some simpler organisms. (3.) Its products are energy rich. (4.) It occurs in the absence of oxygen. (5.) It may occur in the cytoplasm of cells. |
22. 1
|
|
|
23. The main purpose of the citric acid cycle is to
(1.) produce carbon dioxide (2.) produce oxygen (3.) convert glucose to pyruvic acid (4.) produce ATP in abundance (5.) remove energy rich hydrogen from fuels |
23. 5
|
|
|
24. Which process yields the greatest quantity of ATP? (1.) glycolysis
(2.) alcoholic fermentation (3.) citric acid cycle (4.) gluconegenesis (5.) chemiosmotic phosphorylation |
24. 5
|
|
|
25. For its continuation, each turn of the citric acid cycle must conserve one molecule of
(1.) NADH (2.) ATP (3.) citrate (4.) oxaloacetate (5.) acetyl CoA |
25. 4
|
|
|
26. In each turn of the citric acid cycle the total reductions are:
(1.) 3 NAD red and 1 FP red (2.) 5 NAD red and 1 FP red (3.) 6 NAD red and 2 FP red (4.) 8 NAD red and 2 FP red (5.) none of the above |
26. 1
|
|
|
1. The citric acid cycle is involved in the oxidation of glucose. T / F
|
1. T
|
|
|
2. In general, the larger an animal the lower its relative metabolic rate. T / F
|
2. T
|
|
|
3. Cyanide is a poison which blocks the respiratory electron transport chain. T / F
|
3. T
|
|
|
4. The enzymes of glycolysis are membrane bound within the mitochondria. T / F
|
4. F
|
|
|
5. The citric acid cycle and chemiosmosis occur in the mitochondria. T / F
|
5. T
|
|
|
6. Two ATP are produced directly as a result of two turns of the Krebs Cycle. T / F
|
6. T
|
|
|
7. Four (4) ATP of activation energy are required to intiate the glycolysis of a mole of glucose. T / F
|
7. F
|
|
|
8. The net yield of ATP from anaerobic glycolysis is 2 ATP. T / F
|
8. T
|
|
|
9. Glycolysis can operate under either aerobic or anaerobic conditions. T / F
|
9. T
|
|
|
10. The term chemiosmosis reflects the fact that the production of ATP includes both chemical and
transport processes across a selectively permeable membrane. T / F |
10. T
|
|
|
11. Much of the potential energy from glucose becomes tied up in FADH2 and NADH after the Krebs Cycle. T / F
|
11. T
|
|
|
12. Energy carrier molecules are at their lowest energy at the beginning of the electron transport chain. T / F
|
12. F
|
|
|
13. Oxidative phosphorylation chiefly occurs during glycolysis. T / F
|
13. F
|
|
|
14. Anaerobic glycolysis is also called the TCA cycle. T / F
|
14. F
|
|
|
15. Cytochromes are protein structure which hold and pass electrons down the electron transport chain. T / F
|
15. T
|
|
|
16. Protons are pumped from the mitochondrial matrix according to the theory of chemiosmosis. T / F
|
16. T
|
|
|
17. Energy is provided in chemiosmosis by the pH gradient and voltage difference created. T / F
|
17. T
|
|
|
18. The return of protons through channels which allow them to reenter the mitochondrial matrix
provides the energy to convert ADP to ATP. T / F |
18. T
|
|
|
19. ATP synthetase is the enzyme complex which allows the formation of channels in the inner
mitochondrial membrane for protons to return to it. T / F |
19. T
|
|
|
20. The passage of electrons down the electron transport chain appears to provide the energy
for the active transport of protons from the mitochondrial matrix. T / F |
20. T
|
|
|
21. Nearly 40% of the free energy released in glucose oxidation is retained in the form of
newly synthesized ATP molecules. T / F |
21. T
|
|
|
22. The Krebs Cycle and the electron transport chain are anaerobic processes. T / F
|
22. F
|
|
|
23. The net gain of glucose in eukaryotic aerobic respiration is approximately 36 ATP. T / F
|
23. T
|
|
|
24. Glycolysis results in a net yield of 2 ATP and two molecules of NADH. T / F
|
24. T
|
|
|
25. The oxidation of glucose is a chief source of energy in most cells. T / F
|
25. T
|
|
|
26. Lipids yield a much greater amount of energy per gram than carbohydrates or proteins. T / F
|
26. T
|

