![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
167 Cards in this Set
- Front
- Back
|
How do you score a liver biopsy?
|
- Amount of inflammation: "Grade"
- Amount of fibrosis: "Stage" - Both Grade and Stage range from 0-4 (with 0 being no inflammation or fibrosis) |
|
|
How do you assess inflammation on a liver biopsy?
|
Inflammation = Grade:
- 0 = no activity - 1 = minimal - 2 = mild - 3 = moderate - 4 = severe |
|
|
How do you assess fibrosis on a liver biopsy?
|
Fibrosis = Stage:
- 0 = no fibrosis - 1 = portal fibrosis - 2 = periportal fibrosis - 3 = septal fibrosis - 4 = cirrhosis |
|
|
What does the term "cirrhosis" indicate?
|
Stage 4 Fibrosis
|
|
|
What is important to know about the inflammation on a liver biopsy?
|
The "grade" can fluctuate up and down because inflammation is reversible
|
|
|
What does a "grade 1 - minimal activity" score tell you?
|
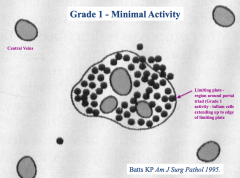
Inflammatory cells are limited to the "limiting plate" - the region around the portal triad
|
|
|
What does a "grade 2 - mild activity" score tell you?
|
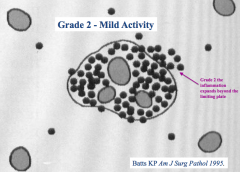
Inflammatory cells extend beyond the "limiting plate" - the region around the portal triad - in one area
|
|
|
What does a "grade 3 - moderate activity" score tell you?
|

Inflammatory cells surround the "limiting plate" - the region around the portal triad
|
|
|
What does a "grade 4 - severe activity" score tell you?
|
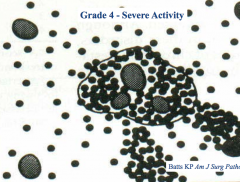
Inflammatory cells spread beyond the area surrounding the "limiting plate" - extend to around the central veins
|
|
|
What does a "stage 1" score tell you?
|
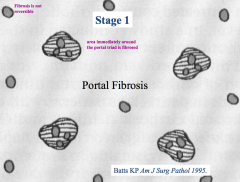
Portal Fibrosis:
- Area immediately surrounding the portal triad is fibrosed |
|
|
What does a "stage 2" score tell you?
|
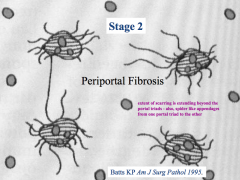
Periportal Fibrosis:
- Extent of scarring is extending beyond the portal triad area - Spider-like appendages extend from one portal triad to another |
|
|
What does a "stage 3" score tell you?
|
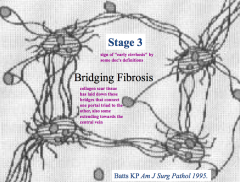
Bridging Fibrosis:
- Collagen scar tissue has laid down bridges that connect one portal triad to the others - Minimal fibrosis towards the central veins |
|
|
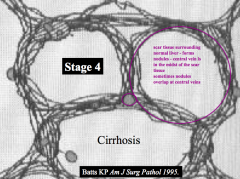
What does a "stage 4" score tell you?
|
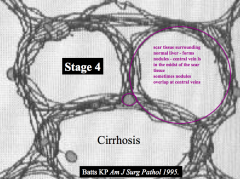
Cirrhosis:
- Scar tissue surrounding normal pockets of liver - Forms "nodules" - Central veins are also covered in fibrosis |
|

What stage of fibrosis is this tissue sample?
|

Stage 0 - very little blue / scar tissue
|
|
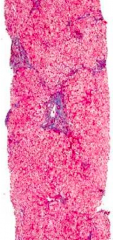
What stage of fibrosis is this tissue sample?
|
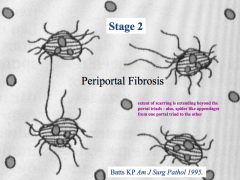
Stage 2 - Periportal Fibrosis
- Scarring around portal triad and starting to extend outwards |
|
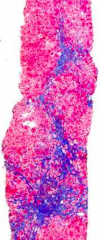
What stage of fibrosis is this tissue sample?
|
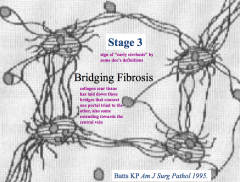
Stage 3 - Bridging Fibrosis
- Starting to form bridges but no nodules |
|
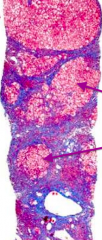
What stage of fibrosis is this tissue sample?
|
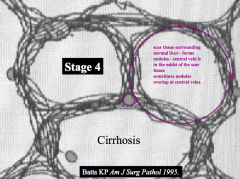
Stage 4 - Cirrhosis
- Presence of nodules |
|
|
How do you test for scarring on a liver biopsy?
|
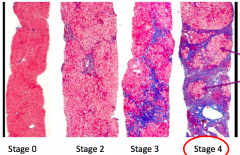
Tri-chome stain (blue = scar tissue)
|
|
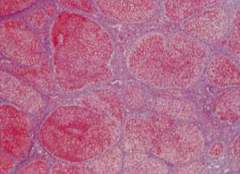
What stage of fibrosis is this tissue sample? What should your next question be?
|
Stage 4 - Cirrhosis
- Presence of nodules *Want to know what their INR is to determine if they are compensating or not. <1.3 is normal* |
|
|
What type of liver damage occurs acutely?
|
Hepatic Necrosis - injury causing immediate cell lysis of hepatocytes
|
|
|
What type of liver damage occurs chronically?
|
Hepatic Fibrosis
|
|
|
What type of liver damage occurs acutely and is complicated by coagulopathy and encephalopathy?
|
Fulminant Liver Failure
|
|
|
What can be the outcome of Hepatic Necrosis?
|
Can lead to acute liver failure
|
|
|
What are the most common causes of acute and fulminant liver failure?
|
- Medications (acetaminophen)
- Viral Hepatitis (but not HepC) |
|
|
What does the term Fulminant Liver Failure mean?
|
Acute liver failure complicated by couagulopathy and encephalopathy
|
|
|
How much alcohol is in an average serving size of beer, wine, and hard liquor?
|

- Beer: 13.8 g
- Wine: 10.7 g - Hard Liquor: 13.4 g |
|
|
What is the definition of "binge drinking" for males and females?
|
- Males: 5+ drinks during a single occasion
- Females: 4+ drinks during a single occasion |
|
|
What is the definition of "heavy drinking" for males and females?
|
- Males: >2 drinks / day on average
- Females: >1 drink / day on average |
|
|
What is "excessive drinking"?
|
Can include heavy drinking, binge drinking, or both
Binge drinking: - Males: 5+ drinks during a single occasion - Females: 4+ drinks during a single occasion Heavy drinking: - Males: >2 drinks / day on average - Females: >1 drink / day on average |
|
|
How is ethanol metabolized?
|
1. Alcohol Dehydrogenase converts Ethanol to Acetaldehyde w/ conversion of NAD+ to NADH (90%)
OR 1. Microsomal Ethanol Oxidizing System (MEOS) uses CYP2E1 to convert Ethanol to Acetaldehyde w/ conversion of NADP+ to NADPH (10%) THEN 2. Acetaldehyde converted to Acetic Acid by Aldehyde Dehydrogenase w/ conversion of NAD+ to NADH |
|
|
What is the function of Alcohol Dehydrogenase?
|
- Converts Ethanol to Acetaldehyde (90%)
- Converts NAD+ to NADH |
|
|
What is the function of the Microsomal Ethanol Oxidizing System (MEOS)?
|
- Converts Ethanol to Acetaldehyde (10%) w/ CYP2E1
- Converts NADP+ to NADPH |
|
|
What is the significance of CYP2E1?
|
- Enzyme that converts 10% of ethanol to acetaldehyde
- Also the enzyme involved in acetaminophen metabolism |
|
|
What is the function of Aldehyde Dehydrogenase?
|
- Converts Acetaldehyde to Acetic Acid
- Converts NAD+ to NADH |
|
|
During alcohol metabolism, what can build up?
|
- NADH
- Acetaldehyde |
|
|
What are the effects of increased NADH in alcohol metabolism?
|
- Inhibits TCA cycle
- Reduces gluconeogenesis - Reduces fatty acid oxidtion |
|
|
What are the effects of increased acetaldehyde in alcohol metabolism?
|
- Activates stellate cells to form collagen (laid down in Space of Disse)
- Microfilaments that maintain intracellular skeleton are sheared leading to ballooning of hepatocytes - Kupffer cells produce TNFα (and IL-8) |
|
|
What cells lay down collagen in the space of Disse during alcohol metabolism? Results?
|
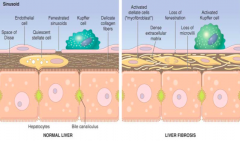
- Stellate Cells → Fibrosis
- Start to lose fenestrations of collagen fibers - Toxins in liver can't be metabolized by hepatocytes because blood isn't interacting with the hepatocytes - Liver proteins can't get out into sinusoids - Start losing microvilli on hepatocytes (poorer absorption) |
|
|
What is the spectrum of disease in Alcoholic Liver Disease?
|
- 90-100% progress to a Fatty Liver
- 10-35% progress from Fatty Liver to Alcoholic Hepatitis - ~40% progress from Alcoholic Hepatitis to Cirrhosis - 8-20% directly progress from Fatty Liver to Cirrhosis |
|
|
What is the first type of damage in Alcoholic Liver Disease? How common? How does this occur?
|
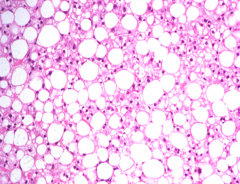
Fatty Liver / Steatosis:
- 90-100% progress from normal liver - Induced by IL-8 and TNF-α (from Kupffer cells) - No inflammatory cells here |
|
|
What is the second type of damage in Alcoholic Liver Disease? How common? How does this occur?
|
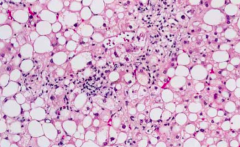
Alcoholic Hepatitis:
- 10-35% of those w/ Fatty Liver - Inflammation induced by IL-8 (from Kupffer cells), IL-8 recruits neutrophils |
|
|
What is the third type of damage in Alcoholic Liver Disease? How common? How does this occur?
|
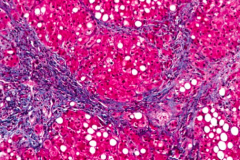
Cirrhosis
- 8-20% directly progress from Fatty Liver - ~40% of those w/ Alcoholic Hepatitis - Scar tissue forms nodules |
|
|
What are the components of the portal triad?
|
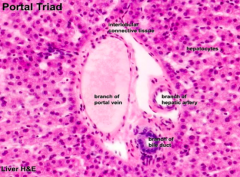
- Branch of portal vein
- Branch of hepatic artery - Branch of bile duct |
|
|
What are the risk factors for Alcoholic Liver Disease?
|
- Quantity of alcohol (>30g/day in men and >20g/day in women)
- Gender (females have greater risk) - Drinking alcohol outside of meals - Binge drinking - Hepatitis C |
|
|
How does a patient with Hepatitis C respond to alcohol?
|
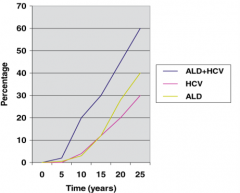
- If you have Hepatitis C, you will have more rapid progression of liver disease with alcohol consumption
- 2 drinks to a person with HepC is more likely equivalent to 5-6 drinks in a healthy person - They can't drink as much because their liver can't metabolize that much alcohol |
|
|
What are the lab abnormalities in Alcoholic Liver Disease?
|
- AST:ALT ratio >2-3
- ALT usually <300 IU/mL - Rarely raised Alk Phos - Low albumin - Prolonged INR (advanced dz) - Macrocytosis / anemia - Thrombocytopenia (advanced dz) |
|
|
What are signs of advanced Alcoholic Liver Disease?
|
- Low albumin
- Prolonged INR - Thrombocytopenia |
|
|
How do you treat Alcoholic Hepatitis?
|
- Abstinence from alcohol and lifestyle modification
- Nutritional support (2000 calories) - Anti-inflammatory drugs (Prednisone or Pentoxifylline-inhibits TNFα) |
|
|
How do you determine if a patient with Alcoholic Hepatitis needs to be on Prednisone or Pentoxifylline?
|
Discriminant function test:
** 4.6 (PT - PT control) + Total Bili If > 32, have a 1-month mortality from 30%-50% so need to be treated! |
|
|
What is the equation for the Discriminant Function Test?
|
4.6 (PT - PT control) + Total Bili
(>32 needs to be treated) |
|
|
Case 1: 38 yo male w/ jaundice and malaise
- ALT 29 - AST 96 - Alk Phos 98 - Total Bili 12 - Direct Bili 8 - PT 19 sec - Control PT 12 sec How should this patient be treated? |
1. They have alcoholic hepatitis d/t the 3:1 ratio of AST:ALT
2. Do Discriminant Testing = 4.6 (PT - PT control) + Total Bili = 4.6 (19-12) + 12 = 44 3. This patient needs to be treated w/ Prednisone or Pentoxifylline (greatly increases chances of survival) |
|
|
What is the mechanism of Pentoxifylline?
|
Inhibits TNFα
|
|
|
What is the 5-year survival in patients with alcoholic cirrhosis?
|
Depends on Child-Turcotte-Pugh score
- Ranges from 20%-60% |
|
|
What is reversible in Alcoholic Liver Disease? What is not reversible?
|
- Steatosis and inflammation are reversible
- Fibrosis/cirrhosis is irreversible |
|
|
What is the most common cause of elevated transaminases in the US?
|
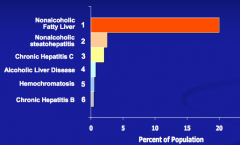
Non-Alcoholic Fatty Liver Disease (NAFLD)
|
|
|
What are the two histologic categories of Non-Alcoholic Fatty Liver Disease (NAFLD)?
|
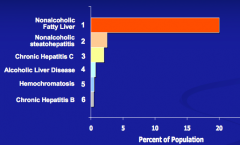
- Non-Alcoholic Fatty Liver (NAFL) - most prevalent
- Non-Alcoholic Steatohepatitis (NASH) - second most prevalent |
|
|
What is the definition of Non-Alcoholic Fatty Liver (NAFL)?
|
Hepatic steatosis WITHOUT inflammation or hepatocellular injury (ballooning of hepatocytes)
|
|
|
What is the definition of Non-Alcoholic Steatohepatitis (NASH)?
|
Hepatic steatosis WITH inflammation and hepatocellular injury (ballooning of hepatocytes) with or without fibrosis
|
|
|
What are the stages of progression of Non-Alcoholic Fatty Liver Disease (NAFLD)?
|
1. Fatty Liver (Hepatosteatosis) → 10% →
2. Steatohepatitis (NASH) → 35% → 3. Steatohepatitis w/ Fibrosis → 15% → 4. Cirrhosis |
|
|
A patient with Non-Alcoholic Fatty Liver Disease (NAFLD) who has a fatty liver (hepatosteatosis) may progress to what stage? How common?
|
10% develop Steatohepatitis (NASH) = inflammation and hepatocellular injury
|
|
|
A patient with Non-Alcoholic Fatty Liver Disease (NAFLD) who has a steatohepatitis (NASH) may progress to what stage? How common?
|
35% develop Steatohepatitis w/ Fibrosis
|
|
|
A patient with Non-Alcoholic Fatty Liver Disease (NAFLD) who has steatohepatitis w/ fibrosis may progress to what stage? How common?
|
15% develop Cirrhosis
|
|
|
How long does it take the progression through the stages of Non-Alcoholic Fatty Liver Disease (NAFLD)?
|
Many years and decades - this is a chronic condition
|
|
|
What are the main risk factors for Non-Alcoholic Fatty Liver Disease (NAFLD)?
|
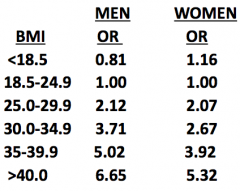
Metabolic Syndrome:
1. Abdominal obesity (BMI >30) 2. High fasting glucose (not necessarily diabetes) 3. Hypertriglyceridemia - Incidence increases w/ age (as does severity of dz) - Males > Females - Hispanics > Caucasians > African Americans |
|
|
How common is Non-Alcoholic Fatty Liver Disease (NAFLD) in patients who are obese?
|
30-100% (probably about 75%)
|
|
|
How common is Non-Alcoholic Fatty Liver Disease (NAFLD) in patients who have diabetes?
|
15-50%
|
|
|
How common is Non-Alcoholic Fatty Liver Disease (NAFLD) in patients who have hypertriglyceridemia?
|
15-80%
|
|
|
Which ethnic groups are at increased risk for Non-Alcoholic Fatty Liver Disease (NAFLD)? Gender?
|
- Hispanics > Caucasians > African Americans
- Males > Females |
|
|
What is the most common liver disease in children in the US?
|
- Non-Alcoholic Fatty Liver Disease (NAFLD)
- NASH is present in 18% of these children |
|
|
How do lipids affect the risk for Non-Alcoholic Fatty Liver Disease (NAFLD)?
|
- Hypertriglyceridemia is a greater risk factor than hypercholesteremia
- 3-fold greater risk w/ TG > 200 |
|
|
What are the causes of steatosis and steatohepatitis?
|
- Alcohol
- Medications: HAART, Amiodarone, Steroids, Tamoxifen, Diltiazem - Nutritional: Total Parenteral Nutrition |
|
|
What are the three stages in the development and progression of Non-Alcoholic Fatty Liver Disease (NAFLD)?
|
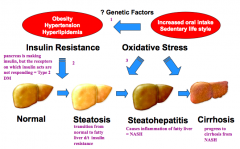
1. Increased oral intake, sedentary lifestyle, genetic factors leads to obesity, HTN, and hyperlipidemia
2. Obesity, HTN, and hyperlipidemia lead to insulin resistance which causes fatty liver changes (steatosis) 3. Oxidative stress causes inflammation (steatohepatitis) and cirrhosis |
|
|
What does a fatty liver look like on ultrasound compared to a normal liver? Limitations?
|
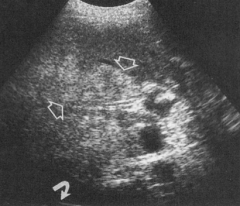
- Normal liver is black
- Fatty liver is bright (arrows) - Can't distinguish from alcoholic fatty liver or NASH, but if there are nodules than it is NASH w/ cirrhosis |
|
|
What does a fatty liver look like on CT compared to a normal liver? Limitations?
|
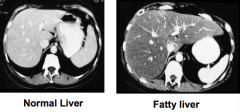
- Normal: spleen and liver are the same color
- Fatty liver: liver is darker than spleen |
|
|
What are the treatment strategies for Non-Alcoholic Fatty Liver Disease (NAFLD)?
|
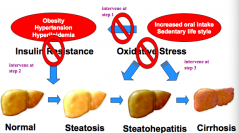
Intervene at the three stages
1. Improve diet/exercise and lose weight 2. Prevent/limit insulin resistance 3. Prevent/limit oxidative stress |
|
|
How does weight loss affect Non-Alcoholic Fatty Liver Disease (NAFLD)? How much weight loss is necessary to show improvements?
|
- Improves liver function tests
- Improves histologic appearance - Lose at least 3-5% of body weight to improve steatosis - Lose at least 10% of body weight to improve inflammation |
|
|
What drugs can be used to treat Non-Alcoholic Steatohepatitis (NASH)?
|
- 1st line: Vitamin E (more effective at improving steatosis, inflammation, ballooning degeneration, transaminases)
- Pioglitazone |
|
|
What are the effects of Pioglitazone and Vitamin E on Non-Alcoholic Steatohepatitis (NASH)?
|
- Both reduced transaminases
- Both reduced inflammation - Neither reduced fibrosis - Vitamin E 43% effective and Pioglitazone 34% effective - Patients receiving pioglitazone gained more weight (4.7 kg) |
|
|
What is Pioglitazone? Use?
|
- Insulin sensitizing agent
- Used to treat biopsy-proven Non-Alcoholic Steatohepatitis (NASH) |
|
|
In what patients is Vitamin E not recommended to treat Non-Alcoholic Steatohepatitis (NASH)?
|
- Diabetic patients
- NAFLD w/o liver biopsy - NASH cirrhosis (damage is irreversible) - Cryptogenic cirrhosis (damage is irreversible) |
|
|
Where is iron absorbed? How much?
|
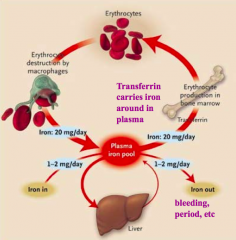
Duodenum - 10-20mg of iron consumed but only 1-2mg absorbed / day
|
|
|
What protein binds Iron and stores it in the liver?
|
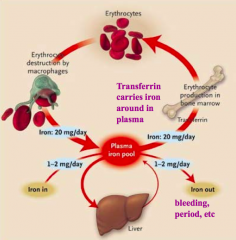
Ferritin
|
|
|
What protein binds Iron in the plasma?
|
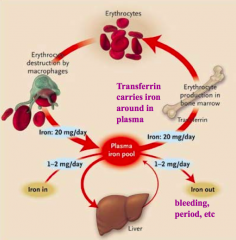
Transferrin
|
|
|
What genes / mutations are responsible for Hereditary Hemochromatosis?
|
HFE gene on chromosome 6:
- C282Y / C282Y: 82-90% of cases - C282Y / H63D: remaining cases (compound heterozygote) |
|
|
What is the function of the HFE gene?
|
- Regulates absorption of iron from small intestine
- Down-regulates transferrin when iron supplies are adequate |
|
|
What happens if there is a defective HFE gene?
|
- Defective HFE gene fails to down-regulate Transferrin
- Iron continues to be absorbed from small intestine - Iron deposits can lead to development of free radicals which can cause organ damage |
|
|
What are some causes of secondary iron overload?
|
- Anemia caused by ineffective hematopoiesis
- Liver disease - Parenteral iron overload |
|
|
What are some causes of primary iron overload?
|
Hereditary Hemochromatosis
|
|
|
Who is affected by Hereditary Hemochromatosis?
|
Most common genetic disease in those w/ European ancestry
|
|
|
What are the combinations of HFE alleles? How common?
|
- Heterozygous state: C282Y/WT (10% in Caucasians)
- Homozygous state: 0.5-1% (C282Y/C282Y) - Compound heterozygote: 3-5% C282Y/H63D |
|
|
What is the clinical presentation of Hereditary Hemochromatosis?
|
- 75% liver function abnormalities
- 74% weakness and lethargy - 70% skin hyperpigmentation (look tan) - 48% diabetes mellitus - 44% arthralgia - 45% impotence in males - 31% EKG abnormalities |
|
|
How does Hereditary Hemochromatosis differ in men and women? Why?
|
Symptoms of Hereditary Hemochromatosis appear at a later age in women d/t menstrual blood loss (which is therapeutic)
|
|
|
How should you evaluate a patient for a possible Hereditary Hemochromatosis diagnosis?
|
1a. Fasting Transferring Saturation
1b. If <45% STOP 1c. If >45% genetic testing 2. C282Y/H63D, C282Y/C282Y, and other, you are checking for |
|
|
If patient is C282Y/H63D what should you do?
|
Consider biopsy
|
|
|
If patient is C282Y/C282Y, what should you do?
|
Biopsy if >40 yo or if elevated ALT/AST
(because you are at greater risk for having developed cirrhosis, which increases your risk of having cancer) |
|
|
If patient has elevated >45% transferrin saturation, what do we do if we don't find a genetic mutation we are looking for (C272Y/H63D or C282Y/C282Y)?
|
Other investigations to determine the cause of secondary iron overload
|
|
|
How do you treat Hemochromatosis? Goals of therapy?
|
Phlebotomy:
- Goal serum Ferritin < 50 - Initially, weekly phlebotomy - Once quarterly thereafter |
|
|
What is the other name for Wilson Disease?
|
Progressive Lenticular Degeneration
|
|
|
What are the dietary sources of copper?
|
- Seafood
- Organ meats - Nuts and nut butters - Legumes - Chocolate - Enriched cereals - Fruits and vegetables |
|
|
How is copper metabolized in the human?
|
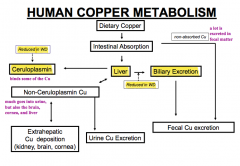
- Some is absorbed in the intestine (the rest is excreted in the fecal matter - a lot)
- Cu absorbed in intestine goes to liver and can be excreted in bile or bound to Ceruloplasmin - Some intestinal copper is also excreted in urine or deposited in kidney, brain, or cornea) - That which is excreted in bile is eventually excreted in fecal matter |
|
|
How does Wilson Disease affect copper metabolism? Implications?
|
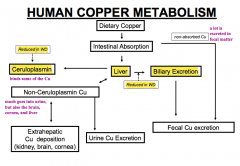
- Decreased biliary excretion of copper from liver
- Decreased Ceruloplasmin to bind copper from liver * Leads to increased non-ceruloplasmin copper (some of which is excreted in urine) others are deposited at extra-hepatic sites such as kidney, brain, and cornea |
|
|
What are the extra-hepatic sites of Cu deposition in Wilson Disease?
|
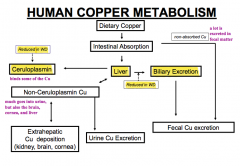
- Kidney
- Brain - Cornea |
|
|
Which protein binds some copper from the liver?
|
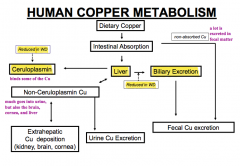
Ceruloplasmin
|
|
|
How do you diagnose Wilson Disease? How common?
|
- Combination of clinical, biochemical, and pathological analysis
- No one single finding is adequate for diagnosis - 30/million |
|
|
When does a diagnosis of Wilson disease occur (age)?
|
Between ages 3-40 (diagnosis of young people - rarely diagnosed in older people because the manifestations of the disease should have already presented)
|
|
|
What disease/symptoms would make you consider Wilson Disease in a patient between 3-40 years?
|
- Unexplained liver disease
* Acute liver failure - Cirrhosis - Neurological symptoms (excess Cu deposits in brain) - Psychiatric symptoms (excess Cu deposits in brain) |
|
|
What are the neurological features of Wilson Disease?
|
• Movement disorders
• Drooling, dysarthria • Rigid dystonia • Dysautonomia • Migraine headaches • Insomnia • Seizures • Depression • Neurotic behavior • Personality changes • Psychosis |
|
|
How do you diagnose Wilson Disease?
|
- Ceruloplasmin: 95% of homozygotes will have level <20 mg/dL
- Note: high CP may occur in inflammation and may lead to false negatives (if high CP doesn't rule out Wilson Disease, but if low it confirms WD) * Urine copper is derived from non-ceruloplasmin copper, rate of excretion in symptomatic patients may exceed 100 µg/24 hours (however then they need to pee in jar for 24 hours which is inconvenient) - Based on copper content in liver (>250 µg/g = homozygous WD and 20-250 µg/g = heterozygous) - Obtain liver biopsy (accumulation of copper will shows as red granules w/ Rhodanine stain) |
|
|
What sign do some patients with Wilson Disease have in their eyes?
|
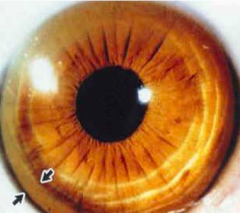
Kayser-Fleischer Rings
|
|
|
What is the concentration of copper in the liver in Wilson Disease? Normal?
|
- Homozygous WD: >250 µg/g dry weight
- Heterozygous WD: 20-250 µg/g dry weight - Normal: 50 µg/g dry weight |
|
|
How do you treat Wilson Disease?
|
- D-Penicillamine
- Trientine - Zinc |
|
|
What is the mode of action of D-Penicillamine? Uses? Concerns?
|
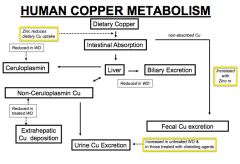
- Binds copper (chelator) and increases urinary excretion of copper (same as Trientine)
- Used to treat Wilson Disease - More side substantial side effects |
|
|
What is the mode of action of Trientine? Uses? Concerns?
|
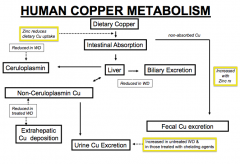
- Binds copper (chelator) and increases urinary excretion of copper (same as D-penicillamine)
- Used to treat Wilson Disease - Better safety profile (fewer side effects) than D-penicillamine |
|
|
What is the mode of action of Zinc? Uses? Concerns?
|
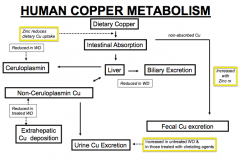
- Prevents copper from being absorbed in GI tract leading to increased excretion of copper in feces
- Used to treat Wilson Disease - Very commonly used (few side effects) |
|
|
What is the most common cause of genetic liver disease in children?
|
α-1 Antitrypsin Deficiency
|
|
|
What is the function of α-1 Antitrypsin? Where is it synthesized?
|
- Inhibitor of proteolytic enzyme Elastase (specifically from neutrophils)
- Synthesized in liver |
|
|
What type of protein is α-1 Antitrypsin? How many alleles?
|
- Serpin (family of protease inhibitors)
- At least 100 alleles of A1AT |
|
|
What is α-1 Antitrypsin Deficiency the most common cause of?
|
- Most common genetic liver disease in children
- Most common genetic disease leading to liver transplantation in children - Most common cause of genetic emphysema in adults |
|
|
What does α-1 Antitrypsin Deficiency cause in adults?
|
- Hepatitis
- Cirrhosis - Liver cancer - Genetic emphysema |
|
|
How is α-1 Antitrypsin deficiency inherited? What are the inherited genes?
|
Autosomal co-dominant
- M (main) - S - Z (most common mutant) |
|
|
What is the normal genotype for individuals with normal A1-AT levels? How common is this in US?
|
- PiMM (Protease Inhibitor w/ two M genes)
- >90% of caucasians in US |
|
|
What organs are affected by α-1 Antitrypsin deficiency? How are they affected?
|
Lung:
- Loss of function - Lack of A1At allows uninhibited PMN medicated proteolytic damage to connective lung tissue - Can lead to genetic emphysema in adults Liver: - Gain of function - Retention of inefficiently secreted A1AT Z molecule in ER triggers hepatotoxic events - Causes hepatitis, cirrhosis, and liver cancer in adults |
|
|
What are the consequences of the different combinations of A1At genotypes (M, S, and Z combinations)?
|
Remember these genes are co-dominant:
- PiMM = 100% activity (no disease) - PiMS = 80% activity (no disease) - PiMZ = 60% activity (no disease) - PiSS = 50-60% activity (no disease) - PiSZ = 30-40% activity (lung disease only) - PiNull-Null = 0% activity (lung disease only) - PiZZ = 10-15% activity (lung and liver disease) |
|
|
Which genotype(s) of α-1 Antitrypsin deficiency causes lung AND liver disease? How much activity of α-1 Antitrypsin?
|
PiZZ - 10-15% activity
|
|
|
Which genotype(s) of α-1 Antitrypsin deficiency causes lung disease ONLY? How much activity of α-1 Antitrypsin?
|
- PiSZ - 30-40% activity
- Pi Null Null - 0% activity |
|
|
How do you diagnose α-1 Antitrypsin deficiency?
|
- Serum A1AT levels
- A1AT phenotypes - Liver biopsy |
|
|
What is the appearance of α-1 Antitrypsin deficiency on histology w/ Periodic Acid-Schiff stain (PAS)?
|
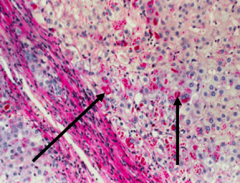
Red accumulations = abnormal A1AT in the liver
|
|
|
Who is affected by Auto-Immune Hepatitis?
|
- Females more commonly (F:M 4:1)
- 50% have other auto-immune disorders |
|
|
What happens in Auto-Immune Hepatitis? How is the onset?
|
- Inflammation of hepatocytes
- Present w/ auto-antibodies in serum - Insidious or acute onset |
|
|
What are the lab values in Auto-Immune Hepatiits?
|
- ALT and AST elevated usually > 1000
- Elevated gamma-globulin (total protein - albumin = globulin) or elevated IgG - Auto-antibodies |
|
|
What characterizes Auto-Immune Hepatitis?
|
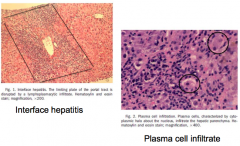
- Interface hepatitis (lymphoplasmacytic infiltrate)
- Portal plasma cell infiltration |
|
|
What are the subclassifications of Auto-Immune Hepatitis?
|
- Type 1: classic, 80-90%, adults
- Type 2: anti-LKM 1 hepatitis, childrens |
|
|
Which type of Auto-Immune Hepatitis is more common in adults? What kind of antibodies are prevalent? What do they target?
|
Type 1 (classic) - 80-90%
- ANA: Anti-Nuclear Antibody - ASMA: Anti-Smooth Muscle Antibody - Nucleus is being targeted by these antibodies |
|
|
Which type of Auto-Immune Hepatitis is more common in children? What kind of antibodies are prevalent? What do they target?
|
Type 2 (anti-LKM 1 hepatitis)
- Anti-liver-kidney-microsomal (LKM) antibody - Microsome is being targeted by these antibodies |
|
|
What associated conditions are there with Auto-Immune Hepatitis? How common?
|
Approximately 50% have other auto-immune disease:
- Thyroid disease - Rheumatoid arthritis - Diabetes - Sjögren's syndrome - Polymyositis - IgA deficiency - Idiopathic thrombocytopenia - Urticaria - Vitiligo - Addison's disease - Inflammatory Bowel Disease - Psoriasis |
|
|
How do you treat patients with Auto-Immune Hepatitis?
|
Immunosuppressive therapy:
- Prednisone - Azathioprine (requires less prednisone) |
|
|
How do you assess whether a patient is in remission from their Auto-Immune Hepatitis?
|
- Normal ALT and AST (can be >1000)
- Normal IgG and gamma globulin (can be elevated) - Resolution of inflammation on biopsy (can have interface hepatitis and portal plasma cell infiltration) |
|
|
What is the response rate to treatment for patients with Auto-Immune Hepatitis? Long-term survival?
|
- 65% respond within 18 months
- 80% respond within 3 years - 20-year survival is 80% |
|
|
What is the mortality rate for patients who are untreated for Auto-Immune Hepatitis?
|
- 50% dead within 3 years
- 90% dead within 10 years |
|
|
What is the definition of Primary Biliary Cirrhosis (PBC)?
|
- Chronic, progressive, CHOLESTATIC liver disease
- Destruction of intrahepatic bile ducts (not visible on imaging) - Probable auto-immune pathogenesis |
|
|
In what disease is there destruction of intrahepatic bile ducts leading to chronic, progressive, cholestatic liver disease? What is the pathogenesis of destruction?
|
Primary Biliary Cirrhosis (PBC)
- Probable auto-immune pathogenesis - High prevalence of serum auto-antibodies (anti-mitochondrial antibodies = AMA, present in 95%) - Elevated IgM |
|
|
Can you detect damage from Primary Biliary Cirrhosis (PBC) on imaging?
|
No because the destruction of intrahepatic bile ducts is microscopic
|
|
|
How common is Primary Biliary Cirrhosis (PBC)? Who is more likely affected?
|
- 25-400 / million cases
- 3500 new cases / year - 90-95% women, predominantly Caucasian - Mean age 52 at presentation (range 30-65) - Often associated with other auto-immune diseases - 6% family history of PBC |
|
|
What antibody is responsible for the damage in Primary Biliary Cirrhosis (PBC)?
|
Anti-mitochondrial antibodies (AMA) - present in 95% of cases
|
|
|
What are the targets of Anti-Mitochondrial Antibodies (AMA) in Primary Biliary Cirrhosis (PBC)?
|
Multi-enzyme complex (pyruvate dehydrogenase complex) = PDC
- PDC-E2 antigen appears to predominate - This Ag is located on the membrane of the biliary epithelial cells |
|
|
What lab tests are seen in Primary Biliary Cirrhosis (PBC)?
|
- Elevated Alkaline Phosphatase
- AMA + (anti-mitochondrial antibody) |
|
|
What are the most common signs and symptoms at presentation of Primary Biliary Cirrhosis (PBC)?
|
- Fatigue (65%)
- Pruritus (55%) because bile deposits in the skin and is very irritating, causing diffuse scratching all over body - 25% are asymptomatic - May also see hepatomegaly, splenomegaly, jaundice, or xanthelasma |
|
|
What is xanthelasma? What disease does it present with?
|
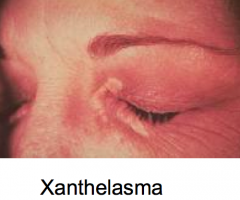
- Cholesterol / lipid deposits underneath the skin, usually on or around the eyelids
- Seen in patients with Primary Biliary Cirrhosis (PBC) |
|
|
What is a xanthomata?
|
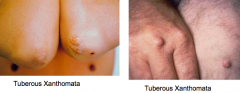
- Irregular yellow patch or nodule on the skin, caused by deposition of lipids
- Seen in patients with Primary Biliary Cirrhosis (PBC) |
|
|
What deficiency is seen in Primary Biliary Cirrhosis (PBC)? Why does this deficiency occur?
|
- Vitamins A, D, E, and K (fat soluble vitamins)
- Decreased absorption because there is decreased bile salt excretion - Severity correlates w/ duration and severity of liver disease |
|
|
What are the effects of Vitamin D deficiency in Primary Biliary Cirrhosis (PBC)? How common is this?
|
8-23%: osteomalacia, fractures, regular bone densitometry, osteoporosis in younger women
|
|
|
What are the effects of Vitamin K deficiency in Primary Biliary Cirrhosis (PBC)? How common is this?
|
7-8%: prolonged PT and therefore long INR
- Even though they don't have hepatocellular disease |
|
|
What kind of liver disease process would giving Vitamin K fix? What wouldn't it?
|
Primary Biliary Cirrhosis (PBC)
- Vitamin K will improve INR because it is caused by vitamin K deficiency - Vitamin K will not fix a hepatocellular cause of elevated INR because here there is a problem synthesizing the clotting factors not d/t deficiency of Vitamin K |
|
|
What are the associated auto-immune disorders in Primary Biliary Cirrhosis (PBC)?
|
- >50% have Sjögren's / Sicca Syndrome (dry eyes and mouth)
- 15% have thyroiditis - 10% have rheumatoid arthritis - <5-10% have scleroderma and CREST syndromes - <5-10% have Raynaud's phenomenon - 6% have Celiac disease |
|
|
What are the lab values in Primary Biliary Cirrhosis (PBC)?
|
- >90% have Alkaline Phosphatase 3-4x normal
- Bilirubin usually rises late - 85% have elevated cholesterol (not associated w/ heart disease) |
|
|
What does Primary Biliary Cirrhosis (PBC) present as histologically?
|
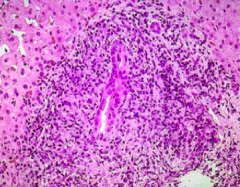
Lymphocytes and mononuclear cells cause inflammatory changes around bile ducts
|
|
|
What is the prognosis for Primary Biliary Cirrhosis (PBC)?
|
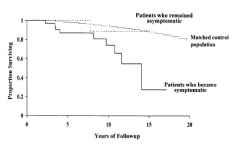
- Patients who remain asymptomatic have a normal lifespan
- Patients who become symptomatic more likely to have premature death (30% survive 15 years) |
|
|
How do you treat Primary Biliary Cirrhosis (PBC)?
|
Ursodeoxycholic Acid (UDCA) - hydrophilic bile acid (used to treat b/c they have elevated hydrophobic bile acids?)
|
|
|
Case 1: 54-yo male presents for evaluation of abnormal liver biochemistries. He is asymptomatic. He consumes 2-3 beers/week and denies illicit drug use or transfusions. Evaluation reveals:
- AST 75 (10-45 U/L) - ALT 71 (8-40 U/L) - Alk phos 96 (40-129 U/L) - Normal total bilirubin - Transferrin saturation 72% (<35%) The next test or treatment in this patient is? |
HFE gene testing
- Genes / mutations are responsible for Hereditary Hemochromatosis - Defective HFE gene fails to down-regulate Transferrin, iron continues to be absorbed from small intestine, and iron deposits can lead to development of free radicals which can cause organ damage |
|
|
Case 2: 37-yo female complains of fatigue. She utilizes artificial tears for dry eyes. She denies alcohol or transfusions. She has multiple sexual partners. Labs:
- AST 51 (10-45 U/L) - ALT 49 (8-40 U/L) - Alkaline phosphatase 812 (40-129 U/L) - Total bilirubin 3.6 (0.2-1.0 mg/dL) - Direct bilirubin 2.3 (0-0.2 mg/dL) What is MOST LIKELY to establish a diagnosis in this patient? |
Testing for Anti-Mitochondrial Antibody
- Liver enzymes show cholestatic injury - Dry eyes and female suggests Sjögren's Syndrome This suggests PBC = Primary Biliary Cirrhosis which is caused by AMA |
|
|
Case 3: 36-yo male presents with generalized malaise and RUQ pain. He consumes alcohol daily. He was recently treated with an oral antibiotic for a upper respiratory tract infection. Labs:
- AST 861 (10-45 U/L) - ALT 430 (8-40 U/L) - Alkaline phosphatase 121 (40-129 U/L) - Total bilirubin 2.6 (0.2-1.0 mg/dL) Which of the following is LEAST LIKELY to explain this patient’s condition? a) HepA b) HepB c) Auto-immune hepatitis d) Medication reaction e) Alcoholic Liver Disease |
Alcoholic Liver Disease
- AST:ALT of >2:1 but the AST and ALT never exceed 300 in alcoholic liver disease, so this is impossible |
|
|
Case 4: 42-yo female presents for evaluation of elevated transaminases.
- AST 613 (10-45 U/L) - ALT 629 (8-40 U/L) Past medical history is remarkable for psoriasis. Liver biopsy reveals interface hepatitis and plasma cells. APPROPRIATE THERAPY in this patient includes which of the following? |
Prednisone (she has auto-immune hepatitis
|
|
|
What is Trientine a treatment for? Mechanism?
|
Wilson's Disease (it is a copper chelator)
|
|
|
What is phlebotomy a treatment for?
|
Hereditary Hemochromatosis
|
|
|
What is Vitamin E a treatment for?
|
NASH: Non-Alcoholic Steatohepatitis
|
|
|
What is Ursodeoxycholic acid a treatment for?
|
Primary Biliary Cirrhosis
|
|
|
Case 5: Which of the following scenarios is consistent with the worst long term prognosis? Normal ranges:
- AST 10-45 U/L - ALT 8-40 U/L - Alkaline phosphatase 40-129 U/L - Total bilirubin 0.2-1.0 mg/dL - INR 0.9-1.3 a) AST 516, ALT 613, Alk Phos 96, total bili 2.6, INR 1.1 b) AST 50, ALT 60, Alk Phos 716, total bili 2.1. INR 1.3 c) AST 25, ALT 20, Alk Phos 60, total bili 0.6, INR 1.0 d) AST 70, ALT 35, Alk Phos 60, total bili 1.6, INR 2.1 e) AST 70, ALT 35, Alk Phos 60, total bili 1.6, INR 1.5 |
d- this has the highest INR
AST 70, ALT 35, Alk Phos 60, total bili 1.6, INR 2.1 |

