![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
58 Cards in this Set
- Front
- Back
|
What are the Rome III criteria for dyspepsia?
|
- Post-prandial fullness (after a meal)
- Early satiety - Epigastric pain or burning |
|
|
What is functional dyspepsia?
|
- Dyspepsia that meets the Rome III criteria (post-prandial fullness, early satiety, and epigastric pain/burning)
* No evidence of structural disease - Symptoms should be fulfilled for the 3 months with symptom onset at least 6 months before diagnosis |
|
|
What are the types of Functional Dyspepsia?
|
- Post-prandial distress syndrome
- Epigastric pain syndrome |
|
|
What are the criteria for Post-prandial Distress Syndrome (functional dyspepsia)?
|
Must include one or both:
- Bothersome post-prandial fullness, after normal sized meals, several times per week - Early satiation that prevents finishing a regular meal, several times per week Supportive: - Upper abdominal bloating or post-prandial nausea or excessive belching - Epigastric pain syndrome may co-exist |
|
|
What are the criteria for Epigastric Pain Syndrome (functional dyspepsia)?
|
All of the following:
- Pain or burning localized to abdomen or chest - Intermittent pain - Not generalized or localized to other abdominal or chest regions - Not relieved by defecation or passage of gas - Not fulfilling criteria for gallbladder or sphincter of Oddi disorders Supportive: - Burning quality but w/o retrosternal component - Pain induced or relieved by ingestion of meal but may occur while fasting |
|
|
What alarm symptoms do you need to look for in patients you are evaluating for dyspepsia?
|
- >55 yo w/ new-onset dyspepsia
- Family hx of upper GI cancer - Unintended weight loss - Progressive dysphagia - Odynophagia (pain swallowing) - Unexplained iron deficiency anemia - Persistent vomiting - Palpable mass or lymphadenopathy - Jaundice |
|
|
How do you evaluate and manage a patient with dyspepsia w/o GERD or NSAIDs that is >55 yo or has alarm symptoms present?
|
Endoscopy
|
|
|
How do you evaluate and manage a patient with dyspepsia w/o GERD or NSAIDs that is <55 yo and has no alarm symptoms?
|
1. Test for H. pylori (if positive, treat)
2. If negative, PPI trial for 8 weeks 3. If H. pylori treatment fails, PPI trial for 8 weeks 4. If fails, reassess diagnosis 5. Consider Endoscopy (if abnormal, biopsies / treatment based on findings) 6. If normal endoscopy, (a) rapid urease test and/or histology for H. pylori; (b) culture sensitivity testing if previously treated for H. pylori --> treat if H. pylori is detected |
|
|
How do you treat Functional Dyspepsia?
|
- Antidepressants (tricyclics, trazodone)
- Prokinetic agents (metoclopramide) - Fundic relaxant drugs (buspirone) - Psychological therapy - Antinociceptive agents (carbamazepine, tramadol, pregabalin) |
|
|
What do you need to do before you consider a diagnosis of functional dyspepsia?
|
Exclude organic causes of dyspepsia (also check for alarm symptoms)
|
|
|
What is the initial management for dyspepsia?
|
- Consider upper endoscopy
- H. pylori testing / treatment - Proton Pump Inhibitor testing for 8 weeks - Consider adjunctive treatments for refractory cases |
|
|
What kind of bacteria is H. pylori?
|
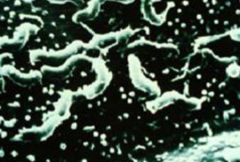
- G-
- Spiral shaped - Microaerophilic - Catalase, oxidase, urease positive - 2-7 flagella |
|
|
What are the things to know about culture H. pylori?
|
- Slow growing
- Culture on blood agar or Skirrow's media at 37 degrees C for 3-7 days |
|
|
What is the benefit of being Urease positive to H. pylori?
|
Urease hydrolyzes luminal urea to ammonia to neutralize the gastric acid
|
|
|
What is the benefit of being Catalase positive to H. pylori?
|
Catalase may protect from oxygen metabolites
|
|
|
Where does H. pylori colonize?
|
- Exclusively colonizes gastric type epithelium
- Non-invasive |
|
|
What happens when H. pylori colonizes the stomach?
|
- Stimulates an inflammatory and immune response
- IL-8 activates neutrophils and recruits other inflammatory cells into the mucosa |
|
|
What are the strains of H. pylori?
|
- CagA positive or negative (Cytotoxin-Associated Gene A)
- If CagA positive, can also be VacA positive or negative (Vacuolating Cytotoxin) |
|
|
What is VacA? Benefit to H. pylori?
|
Vacuolating Cytotoxin
- Passive urea transporter that can create a favorable environment for infectivity - Only expressed in conjunction w/ CagA |
|
|
What is CagA?
|
Cytotoxin-Associated Gene A
|
|
|
Which of the following is true about H. pylori? Fix the rest.
a) gram positive b) catalase +, oxidase +, urease - c) colonizes gastric and duodenal epithelium d) non-invasive organism |
d - non-invasive organism
a - it is G- b - it is ureas + c - it only colonizes gastric epithelium |
|
|
How do you spread H. pylori?
|
- Not known, but likely person to person via fecal/oral or oral/oral exposure
- Humans are the only reservoir |
|
|
If you have had an H. pylori infection and it has been cured, what are your chances of being reinfected?
|
Not typical - only 2% / year
|
|
|
What are the conditions associated with H. pylori?
|
- Gastric adenocarcinoma
- MALT lymphoma (Mucosa-associated lymphoid tissue) - Gastritis - Iron-deficiency anemia w/o bleeding and Vitamin B12 deficiency - Functional dyspepsia? - Acid reflux - Peptic ulcer disease |
|
|
What is the difference between corpus predominant and antrum predominant gastritis associated with H. pylori? How are they affected by treatment?
|
- Corpus predominant: ↓ acid secretion d/t local inflammation and ↑ cytokines, mild worsening w/ treatment
- Antrum predominant: ↑ gastrin level, improves with treatment |
|
|
How does treatment affect corpus predominant gastritis associated with H. pylori?
|
- Treatment helps heal the parietal cells that are damaged by local inflammation and cytokines in this area (corpus)
- Thus once the parietal cells are healthier, they can release more acid which can make the acid reflux symptoms worse |
|
|
How does treatment affect antrum predominant gastritis associated with H. pylori?
|
Treatment improves the acid reflux symptoms
|
|
|
How do you diagnose an H. pylori infection?
|
Endoscopic testing:
- Urease testing (90% sensitivity/specificity) - Histology ± special stains (eg, Giemsa) - Brush cytology (rarely used, 95% s/s) - Bacterial culture (not routine) Non-invasive testing: - Urea breath testing (90% s/s) - Serology - IgG (positive for active and past infections) - Stool antigen (90% s/s) - 13C-urea blood test (rare) |
|
|
How effective is urease testing by endoscopy for diagnosis of H. pylori? False negatives?
|
- 90% sensitivity and specificity
- False negatives: recent bleeding, PPIs, antibiotics, bismuth-containing compounds, H2 antagonists (therefore, if you are taking anything to treat H. pylori or had recent bleeding this may be falsely negative) |
|
|
How effective is histology ± special stains (eg, Giemsa) by endoscopy for diagnosis of H. pylori? False negatives?
|
- Sensitivity may be decreased in patients on anti-secretory therapy (but not as much as for urease testing)
- False negative: potential sampling error (biopsy an area not infected, but could be infected else where) |
|
|
How effective is brush cytology by endoscopy for diagnosis of H. pylori?
|
- Rarely used
- 95% sensitivity and specificity |
|
|
How effective is bacterial culture by endoscopy for diagnosis of H. pylori? Benefits?
|
- Not routine
- Potentially useful for antibiotic sensitivity testing |
|
|
How effective is non-invasive urea breath testing for diagnosis of H. pylori? False negatives?
|
- Detect labeled CO2 and ammonia with hydrolysis of urea
- 90% sensitivity and specificity - False negatives: PPIs, bismuth, and antibiotics - Useful for initial diagnosis and confirmation of eradication |
|
|
What is the test of choice for confirming eradication of H. pylori?
|
Urea Breath Testing (detect labeled CO2 and ammonia w/ hydrolysis of urea)
|
|
|
How effective is non-invasive serology (IgG) for diagnosis of H. pylori? Usefulness?
|
- High sensitivity but variable specificity (76-96%)
- Positive result represents an active infection only about 50% of the time - Negative test result useful to exclude infection in a low pretest probability situation - Cure if a positive test converts to a negative after treatment (not common) |
|
|
How effective is non-invasive stool antigen testing for diagnosis of H. pylori? False negatives?
|
- 90% sensitivity and specificity (but not as good as urea breath test)
- Useful for initial diagnosis and documenting successful eradication (may be less accurate for latter) - Higher false negative result w/ PPI or bismuth |
|
|
What tests can be used to document successful eradication of H. pylori infection?
|
- Serology (IgG) testing
- Stool Antigen testing |
|
|
How effective is non-invasive 13C-urea blood testing for diagnosis of H. pylori? How?
|
- Rarely used
- Serum testing before and after ingestion of a 13C-urea rich meal |
|
|
How do you treat H. pylori gastritis?
|
- Antibiotics (eg, amoxicillin and clarithromycin)
- Acid suppression w/ PPIs - Treat for 10-14 days |
|
|
What is the pathogenesis of NSAID gastroduodenal toxicity?
|
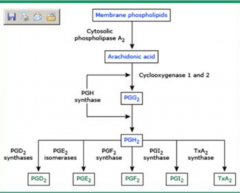
- COX-1 constitutively expressed to produce prostaglandins but NSAIDs inhibit COX-1
- Prostaglandins are cytoprotective |
|
|
What are the cytoprotective effects of prostaglandins?
|
- Stimulates mucin, bicarbonate, and phospholipid secretion
- Increases mucosal blood flow and O2 delivery - Enhances epithelial cell proliferation - Increases epithelial cell migration |
|
|
What are the cytoprotective effects of NO through NO Synthase?
|
- Mediates gastric mucin release
- Stimulation of alkaline fluid - Maintenance of epithelial barrier - Increases mucosal blood flow |
|
|
What are the general repair mechanisms for gastroduodenal toxicity?
|
- Restitution: rapid migration of new epithelial cells from progenitor cells
- Proliferation: less rapid regeneration of new epithelial cells from progenitor cells - Prostaglandins and NO important in both |
|
|
What is the term for rapid migration of new epithelial cells from progenitor cells to repair the gastroduodenal epithelium?
|
Restitution
|
|
|
What is the term for less rapid regeneration of new epithelial cells from progenitor cells to repair the gastroduodenal epithelium?
|
Proliferation
|
|
|
What contributes to the pathogenesis of gastroduodenal toxicity by NSAIDs?
a) ↑ COX-1 activity b) ↓ COX-2 activity c) ↑ NOS activity d) ↓ Phospholipid secretion |
d) ↓ phospholipid secretion
(COX-1 is the main enzyme affecting the GI, so ↓ COX-1 would also have been correct; ↓ NOS activity would also mediate the gastroduodenal toxicity) |
|
|
How does NSAID use relate to H. pylori?
|
- They are independent and synergistic risk factors for PUD
- ↓ Risk of PUD w/ H. pylori eradication before starting NSAIDs |
|
|
What are the risk factors for PUD with NSAID use?
|
- Duration of therapy (most commonly complications occur in first 3 months)
- Higher dose - Age - Hx of gastroduodenal toxicity to NSAIDs - Hx of PUD - Glucocorticoids, anticoagulation, clopidogrel, bisophosphonates, SSRIs |
|
|
Are dyspepsia and GI toxicity from NSAIDs related?
|
No
|
|
|
How long does it take to recover COX-1 activity after aspirin is stopped?
|
5-8 days (remember aspirin is an irreversible acetylator of COX-1)
|
|
|
How does NSAID use affect the stomach vs the duodenum?
|
Stomach
- Interference of NSAIDs w/ restitution and proliferation in stomach - Ulcer formation less dependent on gastric acid than the COX-1 mediated prostaglandin production Duodenum - Ulcer formation w/ NSAIDS highly dependent on stomach acid |
|
|
How do PPIs and H2 blockers help the stomach vs the duodenum in NSAID GI toxicity?
|
H2 blockers and PPIs are more effective in preventing injury in the duodenum than in the stomach
|
|
|
How can NSAIDs affect the distal small intestine and colon?
|
- High local concentration of NSAID necessary which could be exacerbated by enteric-coated, sustained-release, or slow-release NSAIDs
- Localized mucosal injury → inflammation and ulceration → fibrosis and stricture - NSAIDs inhibit prostaglandins, alter blood flow, and increase small intestinal permeability |
|
|
What is the clinical presentation of NSAID related GI toxicity to the stomach and duodenum?
|
- Edema, erythema, subepithelial hemorrhage
- Erosions (mucosal break w/o visible depth) - Ulcers (mucosal breaks w/ visible depth) |
|
|
Which is deeper an erosion or an ulcer?
|
Ulcer
|
|
|
What is the clinical presentation of NSAID related GI toxicity to the distal small intestine and colon?
|
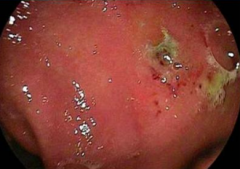
- Iron deficiency anemia
- Overt bleeding from ulcers (picture) - Hypoalbuminemia - Malabsorption from enteropathy - Bowel obstruction from strictures (eg, diaphragm) - Diarrhea from colitis - Acute abdomen from perforation |
|
|
How do you treat NSAID related GI toxicity to the stomach and duodenum?
|
- Stop NSAID
- Start PPI >> H2 antagonist (4 weeks for duodenal ulcers and 8 weeks for gastric ulcers b/c more effective in duodenum) - Assess H. pylori status and treat if positive |
|
|
How do you treat NSAID related GI toxicity to the distal small intestine and colon?
|
- Stop NSAID
- Dilation for strictures - Surgery |

