![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
25 Cards in this Set
- Front
- Back
|
In order for Transcription Factors to activate or repress transcription they must have what 3 features?
|
1. Be located in the nucleus
2. Bind to DNA and/or 3. Interact with the basal transcriptional apparatus |
|
|
If an extracellular signal wants to effect Transcription Factor activity, what must it regulate?
|
The Nuclear Localization, DNA Binding, and/or Transactivation.
A given signal doesn't necessarily have to do all 3 modes of regulation, just one will do. |
|
|
Regulation of Transcription Factor activity: Protein Synthese
|
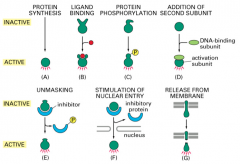
only make the protein when it is needed and quickly degrade it afterwards so that it does not accumulate and cause unwanted effects.
|
|
|
Regulation of Transcription Factor activity: Ligand Binding
|
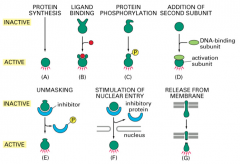
Think about Steroid Hormone Receptors. If a glucocorticoid binds the glucocorticoid receptor, there is a conformational change, the hormone bound receptor dimerizes with another hormone bound receptor, a NLS and DNA binding domain are revealed, and the complex moves to the nucleus to affect transcription.
|
|
|
Regulation of Transcription Factor activity: Covalent Modification
|
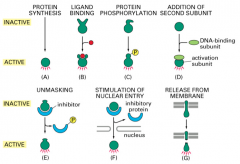
activation by covalent modification, including phosphorylation*
|
|
|
Regulation of Transcription Factor activity: Unmasking
|
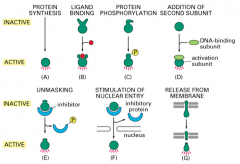
this is when an inhibitor is released from the transcription factor, allowing the TF to be active. This is common for tumor suppressors.
|
|
|
Regulation of Transcription Factor activity: stimulation of nuclear entry
|
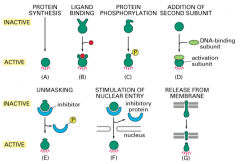
this is an example where the inhibitor is removed to reveal a NLS and the protein can then permeate the nucleus
|
|
|
Regulation of Transcription Factor activity: release from the membrane
|
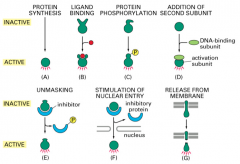
a protein is in the membrane and undergoes regulated proteolysis to release an active portion.
|
|
|
nuclear localization pathwa
|
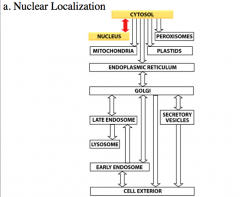
the protein is synthesized on soluble ribosomes in the cytoplasm and then the protein gets imported into the nucleus
|
|
|
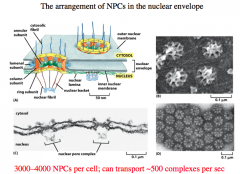
Nuclear Envelope
|
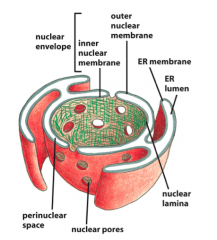
-double membrane
-penetrated with pores -continuous with Endoplasmic Reticulum -permeated with thousands of nuclear pores, each which can transport ~500 complexes per second -nuclear lamina is a fibrous meshwork underlying the inner membrane |
|
|
Nuclear Pore Complex
|
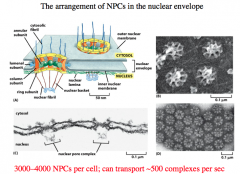
Made up of 30 distinct proteins and many natively unfolded proteins.
The inner pore is made up of annular proteins and column proteins. There is a luminal subunit that holds the pore in place. The Cystolic Fibrils protrude into the cytoplasm and guide the proteins into the NPC. Other fibrils on the nuclear side of the pore give rise to a basket. NPC serves as a *Gated Diffusion Barrier* -small molecules can freely diffuse through to the nucleus. -larger molecules (greater than 60,000 Da) need "help" and are actively transported. |
|
|
Gated Diffusion Barrier of the NPC
|
The hydrophobic residues of the core are there but not highly interacting.
These "noodles" can be pushed aside by cargo-importer complexes but keep other things out. |
|
|
Nuclear Localization Signal
|
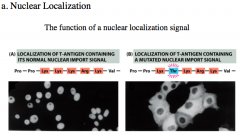
The NLS consists of a lot of basic amino acids like Lysine and Arginine.
Required for active transport of large molecules. NLS on a protein is essential for transporting the protein into the nucleus. If there is a mutation, or if the NLS is "hidden" (and requires a conformation change or dimerization for it to be "revealed"), the protein cannot get into the nucleus to affect transcription, and thus remains in the cytoplasm. |
|
|
Nuclear Import Receptors
|
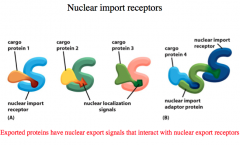
There are many players involved in getting the transcription factor into the nucleus. The protein is called "cargo" There are dozens of Nuclear Import Receptors that can either:
a.) bind the cargo via its NLS b.) bind an adaptor protein --the adaptor protein is similar to the Nuclear import receptor because it recognizes NLS on proteins. THerefore, it binds the NLS on the cargo, and then it has its OWN NLS by which the Nuclear Import Protein ("import in") binds, transporting the adaptor+Cargo into the nucleus. |
|
|
Ran
|
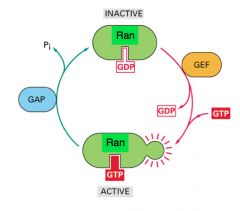
small GTPase
Main regulatory protein that functions in the transport of RNA and protein into and out of the nucleus. It uses the conformational changes associated with the binding of GTP and GDP to bind and release cargo. It has very weak endogenous GTPase activity, and is instead governed by GAP and GEF. Active when bound to GTP. Inactive when bound to GDP. |
|
|
GTPase Activating Protein (GAP)
|
GAP accelerates the intrinsic GTPase activity.
Ran-GAP promotes the dephospho relation of the bound GTP to GDP, and is thus an inhibitory protein. *Ran-GAP is located primarily in the cytoplasm. |
|
|
Guanine Nucleotide Exchange Factor (GEF)
|
Ran-GEF promotes the dissociation of GDP and the binding of GTP, and is therefore an activating protein.
*Ran-GEF is located primarily in the nucleus. |
|
|
What stimulates Ran?
|
1. Inhibition of GAP
2. Activation of GEF 3. Inhibition of GDI -GDI stabilizes the inactive Ran-GDP state |
|
|
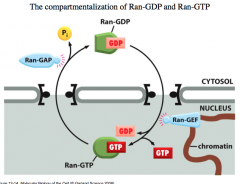
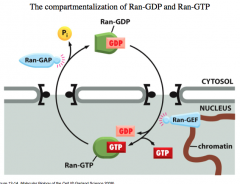
"Gradient" of Ran-GDP/GTP
|
In the cytosol, GAP is high and this means that it enhances the GTPase activity on Ran, effectively causing Ran-GTP to switch to Ran-GDP.
In the nucleus, GEF is high so it causes GDP to dissociate from RAN, opening up a high affinity binding site for GTP. -the dissociation of GDP is the rate limiting step of the cycle. GEF speeds this step up and is therefore an activating protein for nuclear localization. This whole process effectively gives *directionality* to nuclear transport. |
|
|
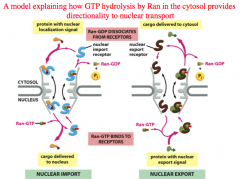
Ran-GTP and Cargo Release
|
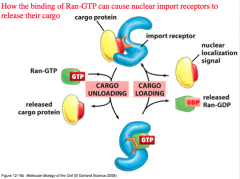
1. The protein with its exposed NLS binds to the import receptor.
2. a.) The Importin+Cargo move through the NPC through interaction of the FG repeats on the NPC b.) In the mean time, Ran-GDP binds to its own Nuclear Import Receptor, which is specific for Ran-GDP (not GTP!) and brings it into the nucleus. Remember that there is a lot of GAP in the cytoplasm, which is why there is a lot of Ran-GDP that can be brought into the nucleus. 3. Inside the nucleus we see GEF, which causes the Ran-GTP form to predominate. *Ran-GTP competes with the cargo for binding on the Nuclear Import Receptor*. Ran-GTP outcompetes the cargo, leaving the cargo behind (MISSION ACCOMPLISHED! Now the protein is inside the nucleus!) |
|
|
What governs nuclear localization and the directionality of nuclear transport?
|
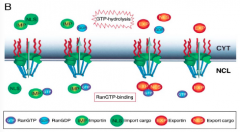
Ran and its two forms: Ran-GTP versus Ran-GDP
Ran-GTP is mostly in the nucleus because of the high concentration of GEF and is required to compete with the cargo for binding on the nuclear import receptor that just brought the cargo through to the nucleus. Ran-GTP is brought out to the cytoplasm along with the importin receptor, the GTP is hydrolyzed (GAP is helping), causing Ran-GDP and the importin to dissociate from each other, starting the process all over again. |
|
|
Interior of Pore Complex
|
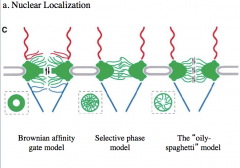
Gel-like interior
-created by taking the proteins from the center of the nuclear pore complex and repeating the repeat domain The inner nuclear membrane has membrane proteins that were synthesized on the ER membrane. We regulate access to the nucleus by regulating access to the NLS. An example of this is the glucocorticoid receptor. Hormone receptors are both receptors and transcription factors, and in the case of the glucocorticoid receptor, when it is not bound to glucocorticoids, it is found in the cytoplasm. When glucocorticoids bind the receptor it causes a conformational change that no only creates DNA binding domains but also reveals and NLS so now the activated complex can be transported through the pore and interact with the DNA. |
|
|
DNA Binding
|
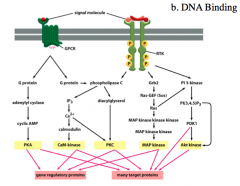
*The 2nd Method of Regulating Transcription Factors*
In order to regulate DNA binding, the proteins have to be in the nucleus. Targets of the kinases that are the end products of the intracellular signaling pathways are often transcription factors. These transcription factors can be phosphorylated and now have access to the nucleus to bind DNA. The DNA binding positions the protein for its transactivation domain to properly interact with other transcription proteins. The main point is that some transcription factors require modification, such as phosphorylation, to bind to DNA. |
|
|
DNA Binding Example: JAK-STAT Signaling Pathway
|
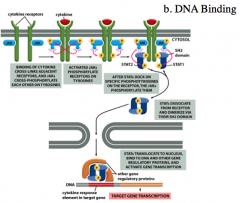
-The JAK-STAT signaling pathway is activated by cytokines.
-The Tyrosine Kinase Receptors dimerize when a cytokine binds and become autophosphorylated. -This creates the SH2 binding domains that bind to proteins. -STAT will then come and bind to the phosphorylated receptor at which point the JAKs will phosphorylate them. -Once phosphorylated, STAT will dissociate from the receptor, dimerize through the SH2 binding domains and then translocate to the nucleus where they will bind to DNA. *The dimerization of STATs causes a conformational change that reveals the NLS and DNA binding domain. The STATs bind the DNA and interact with other regulatory proteins to activate transcription of the target gene. |
|
|
Transactivation
|
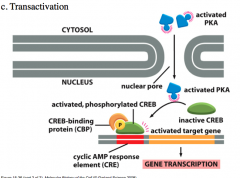
*3rd Method of Regulating Transcription Factors*
Transactivation is important because proteins bind to DNA with seemingly low specificity. Transactivation is required so that the proteins that should have an impact on DNA and gene expression do. Activated PKA molecules must have a newly exposed NLS that allows them to travel into the nucleus. Main point is that some transcription factors can bind to DNA, but cannot participate in gene expression unless they are acted on by a transactivator. |

