![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
240 Cards in this Set
- Front
- Back
|
Describe the break down of the Eukaryotic microogranism:
|
Fungi
Protists: Algae and Protozoa |
|
|
Define Cytoskeleton
|
provides structure and shape of cell, three
components: Microtubules Microfilaments Intermediate filaments |
|
|
Define Intermediate filaments
|
Intermediate filaments - very stable structural element, play
a supportive role, composed of proteins including keratin. |
|
|
Define Microfilaments
|
Microfilaments - smallest element of cytoskeleton, composed
of actin, involved in motion (pseudopod formation). |
|
|
Define Microtubules
|
Microtubules - largest element of cytoskeleton, composed of
hollow cylinders of tubulin, form mitotic spindles, cilia and flagella and cell “highways”. |
|
|
Practice: Microfilaments
a. are a component of the cytoskeleton. b. are long, twisted polymers of a protein called actin. c. form eukaryotic flagella. d. are made of tubulin. e. a and b f. c and d |
E. a and b
Microfilaments a. are a component of the cytoskeleton. b. are long, twisted polymers of a protein called actin. |
|
|
Define Nucleus
|
Nucleus
Bound by both an inner and outer membrane. The space between the two membranes is called the perinuclear space. The membrane has large nuclear pores through which proteins can pass. |
|
|
What is the space between the two membranes of the Nucleus called?
|
Perniuclear Space
|
|
|
Linear pieces of DNA are packaged by wrapping one and three quarters times around a _________ to form a core particle.
|
histone octamer
|
|
|
What is the space between the two membranes of the Nucleus called?
|
Perniuclear Space
|
|
|
What forms a nuceosome?
|
Core Particle, H1, and some of the linker DNA form a nucleosome.
|
|
|
Histones are rich in what type of amino acid?
|
Lysine
|
|
|
How does the charge of DNA/histones affect the packaging process?
|
DNA: - negative charge
Histones: + positive charge Charge interactions |
|
|
What are chromatin?
|
Chromatin is the complex of DNA and proteins that together form the chromosomes.
|
|
|
How does euchromatic differ from heterochromatin?
|
Euchromatic: DNA relaxed, genes here are actively expressed.
Heterochromatin - tightly coiled, genes are not actively expressed most of the time. |
|
|
Define Heterochromatin:
|
Heterochromatin - tightly coiled, genes are not actively expressed most of the time.
|
|
|
Define Euchromatic
|
Euchromatic: DNA relaxed, genes here are actively expressed.
|
|
|
Which region within the nucleus is the site of ribosome assembly?
|
Nucleolus
|
|
|
Practice: The nucleus
a. is a double membrane sac containing DNA as is found in eukaryotes. b. Is a single phospholipid membrane sac containing prokaryotic DNA. c. is a smaller structure contained within the eukaryotic nucleolus. d. cannot transport molecules to the cytoplasm due to the double membrane barrier. |
a. is a double membrane sac containing DNA as is
found in eukaryotes. |
|
|
The Endoplasmic Reticulum consists of?
|
Rough ER: protein
Smooth ER: lipid *vesicles bud off of both of these organelles carrying the recently synthesized compounds. These vesicles are targeted for the Golgi. |
|
|
Define Rough ER:
|
Rough - Protein
Site of synthesis of proteins targeted for the membrane, for secretion or for specific organelles. (Studded with 80S ribosomes (*60S and 40S subunits)) |
|
|
Define the Smooth ER
|
Smooth - Lipid
Smooth ER - Site of lipid and steroid hormone synthesis, location of calcium ion storage. (No ribosomes cover the surface, thus smooth) |
|
|
After the vesciles have budded off of the smooth or rough ER, the vesicles are targeted for what organelle?
|
Golgi Apparatus
|
|
|
Practice: Which one of the following would be the site of
synthesis and folding, but not final modification of a protein needed outside the cell? a. The Golgi apparatus b. The mitochondria c. The smooth ER d. The rough ER |
d. The rough ER
|
|
|
Define the Golgi apparatus
|
The Golgi apparatus - molecules from ER are further modified
(e.g. addition of carbohydrate or phosphate groups). Vesicles bud off of Golgi and carry modified molecules to their destination. The Golgi has a cis and a trans face that differ substantially from one another. |
|
|
Practice: The Golgi Apparatus
a. is the site of modification of molecules from the ER. b. has an acidic pH and is filled with hydrolytic, digestive enzymes. c. is found exclusively in plants and algae. d. is considered the “powerhouse” of the eukaryotic cell. e. both b and c |
a. is the site of modification of molecules from the ER.
|
|
|
True or False: New ER is synthesized from old.
|
True
|
|
|
Nuclear Envelope consists of which two components?
|
Outer nuclear membrane
Inner nuclear membrane |
|
|
Which site is the location of rRNA synthesis, the site of partial assembly of ribosomes?
|
Nucleolous
|
|
|
Define Lysosomes.
|
(recyclers)
Lysosomes are cellular organelles that contain acid hydrolase enzymes to break down waste materials and cellular debris They are found in animal cells, while in yeast and plants the same roles are performed by lytic vacuoles.[1] Lysosomes digest excess or worn-out organelles, food particles, and engulf viruses or bacteria. The membrane around a lysosome allows the digestive enzymes to work at the 4.5 pH they require. At pH 4.8, the interior of the lysosomes is acidic compared to the slightly alkaline cytosol (pH 7.2). The lysosome maintains this pH differential by pumping protons (H+ ions) from the cytosol across the membrane via proton pumps and chloride ion channels. The lysosomal membrane protects the cytosol, and therefore the rest of the cell, from the degradative enzymes within the lysosome |
|
|
Define Mitochondria.
|
Powerhouse of the cell
Mitochondria are rod-shaped organelles that can be considered the power generators of the cell, converting oxygen and nutrients into adenosine triphosphate (ATP) |
|
|
Practice: Draw a Mitochondria, label all parts.
|
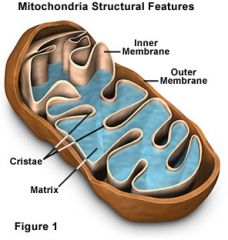
|
|
|
Practice - Which one of the following organelles contains its own
DNA genome and 70S ribosomes? a. lysosomes b. the endoplasmic c. mitochondria |
c. mitochondria
|
|
|
Which statement/s regarding mitochondria is/are FALSE?
a. Mitochondria are capable of converting CO2 into organic compounds. b. Mitochondria reproduce by binary fission. c. Nearly all eukaryotic cells contain mitochondria. d. Mitochondria contain their own DNA genome |
a. Mitochondria are capable of converting CO2 into
|
|
|
Define Chloroplasts.
|
Chloroplasts are organelles found in plant cells and other eukaryotic organisms that conduct photosynthesis. Chloroplasts capture light energy to conserve free energy in the form of ATP and reduce NADP to NADPH through a complex set of processes called photosynthesis
|
|
|
Practice - draw and label all parts of a Chloroplast
|
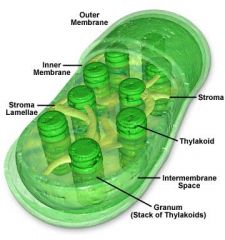
|
|
|
Where are the components of the electron tranport chain in a Mitochondria?
|
Inner Membrane of the Cristae
In mitochondria, it is the conversion of oxygen to water, NADH to NAD+ and succinate to fumarate that generates a proton gradient |
|
|
Where are the components of the electron tranport chain in Chloroplasts?
|
Thylakoid membrane
In chloroplasts, light drives the conversion of water to oxygen and NADP+ to NADPH with transfer of H+ ions across chloroplast membranes |
|
|
Where are the components of the electron tranport chain in prokaryotes?
|
Cell membrane
|
|
|
Practice - Both chloroplasts and mitochondria synthesize ATP
using the electron transport chain, in what ways are these two organelles different (in terms of the origin of the electrons that fuel the electron transport chain)? |
In chloroplasts, light drives the conversion of water to oxygen and NADP+ to NADPH with transfer of H+ ions across chloroplast membranes.
In mitochondria, it is the conversion of oxygen to water, NADH to NAD+ and succinate to fumarate that generates a proton gradient |
|
|
Practice: Which of the following explains the origin of
mitochondria and chloroplasts? a. The Chemiosmotic Hypothesis b. The Endosymbiont Theory c. The Fluid Mosaic Model d. Spontaneous generation |
b. The Endosymbiont Theory
|
|
|
True or False.
In many ways the plasma membrane of a eukaryotic cell is similar to that of a prokaryotic cell (e.g Both are composed of a semipermeable phospholipid bilayer (fluid-mosaic). Both contain transport proteins. |
True.
|
|
|
Practice - List the ways in which the plasma membrane of a
eukaryotic cell is different than that of a prokaryotic cell. |
Processes such as oxidative phosphorylation and photosynthesis take place across the prokaryotic plasma membrane, instead of chloroplasts and mitochondria of eukaryotic cells.
Eukaryotes: Membranes are assymetic - lipids and proteins on the inner leaflet are very different from those on the outer leaflet. Membranes contain sterols (cholesterol in animal cells) that control membrane fluidity and add strength. Contain more glycoproteins that function as receptors (cell to cell singalling) as well as glycolipids. |
|
|
Define Endocytosis.
|
Endocytosis is a process by which cells absorb molecules (such as proteins) by engulfing them. It is used by all cells of the body because most substances important to them are large polar molecules that cannot pass through the hydrophobic plasma or cell membrane
http://www.youtube.com/watch?v=4gLtk8Yc1Zc |
|
|
List the two types of Endocytosis.
|
Endocytosis
Pinocytosis |
|
|
Define Phagocytosis.
|
Phagocytosis (from Ancient Greek φαγεῖν (phagein) , meaning "to devour", κύτος, (kytos) , meaning " cell", and -osis, meaning "process") is the cellular process of engulfing solid particles by the cell membrane to form an internal phagosome by phagocytes and protists
cell sends out long extensions called pseudopods. These surround debris and form a phagosome which fuses with a lysosome to form a phagolysosome http://www.youtube.com/watch?v=7VQU28itVVw |
|
|
Define Pinocytosis.
|
pinocytosis ("cell-drinking", "bulk-phase pinocytosis", "non-specific, non-absorptive pinocytosis", "fluid endocytosis") is a form of endocytosis in which small particles are brought into the cell - forming an invagination, and then suspended within small vesicles that subsequently fuse with lysosomes to hydrolyze, or to break down, the particles. This process requires a lot of energy in the form of adenosine triphosphate, the chemical compound used as energy in the majority of cells. Pinocytosis is used primarily for the absorption of extracellular fluids (ECF),
|
|
|
List the 3 types of Pinocytosis.
|
a.) Macropinocytosis
b.) Clathrin-dependent (Receptor mediated) c.) caveolae-forming |
|
|
How many sacs of cisternae are there per stack within a Golgi Apparatus?
|
4-9 sacs/ stack
|
|
|
A stack of cisternae are called?
|
Dicytosome
|
|
|
Vescles leave the Golgi targeted for what locations?
|
Lysosomes, the cell membrane, or outside of the cell (also known as the biosynthetic-secretory pathway)
|
|
|
True or False.
Although Golgi Apparatus can be observed in most eukaryotic cells, it is not well-fromed in many fungi and ciliate protists. |
True.
|
|
|
Which side do vesicles enter the Golgi Apparatus?
Which side do vesicles bud off of the Golgi Apparatus? |
Enter - Cis
Exit - Trans |
|
|
What is the pH range of the acidic interior of a Lysosome?
|
pH: 3-5
|
|
|
What's unique about Mitochondria?
|
They contain their own DNA genome, enzymes to replicate genome, and 70S ribosomes.
|
|
|
Define Macropinocytosis.
|
Cell invaginates to form a large fluid-filled vescicle. Process is nonselective.
|
|
|
Define Clathrin-dependent pinocytosis.
|
(Receptor mediated)
A specific molecule binds to an external receptor of a clathrin-coated pit. A bit of fluid, as well as the bound molecule are taken into the cell. |
|
|
Define Caveolae-forming pinocytosis.
|
A piece of membrane is pinched off to form a small invagination that is rich in cholesterol and the protein caveolin. Acine in the import of folic acid and other macromolecules. Caveolae don't fuse/ lysosomes., and thus toxins, viruses, bactera, and protozoa often enter this way.
|
|
|
How do toxins, viruses, bactera, and protozoa often enter a cell?
|
via Caveolae-forming pinocytosis.
|
|
|
Define Exocytosis
|
http://www.youtube.com/watch?NR=1&v=1w10R9lv7eQ
Membrane vesciles inside the cell fuse with the plasma membrane their contents are released. Exocytosis (/ˌɛksoʊsaɪˈtoʊsɪs/; from Greek ἔξω "out" and English cyto- "cell" from Gk. κύτος "receptacle"), also known as 'reverse pino-cytosis', is the durable process by which a cell directs the contents of secretory vesicles out of the cell membrane. These membrane-bound vesicles contain soluble proteins to be secreted to the extracellular environment, as well as membrane proteins and lipids that are sent to become components of the cell membrane. |
|
|
Practice: Which statement/s is/are TRUE?
a. Phagocytosis and pinocytosis are both types of endocytosis. b. Receptor mediated (clathrin-dependent) endocytosis is a type of pinocytosis. c. During phagocytosis, a phagolysosome is formed. d. During caveolae-forming endocytosis, no lysosome fusion occurs, thus many pathogens enter cells this way. e. all of the above |
e. all of the above
|
|
|
True or False.
Meany pathogens exploit endocytosis to enter a host cell. |
True
|
|
|
Practice: How are flagella different in prokaryotes and
eukaryotes? All of the following are true of eukaryotic flagella EXCEPT a. eukaryotic flagella are composed of 9 pairs of microtubule doublets. b. eukaryotic flagella sometimes have lateral hairs called flimmer filaments. c. eukaryotic flagella are composed of a basal body, a hook and a filament. d. eukaryotic flagella function in motility by wave-like motion (base to the tip or tip to the base). |
c. eukaryotic flagella are composed of a basal body, a hook and a
filament. |
|
|
Practice: Cilia
a. are also called pili. b. are also called fimbriae. c. are shorter than flagella. d. move in two phases. e. a and b f. c and d |
f. c and d
c. are shorter than flagella. d. move in two phases. |
|
|
What are Eukaryotic flagella composed of?
|
9 pairs of microtubule doublets that surround a central tubules. This is called the 9 + 2 pattern.
|
|
|
What is the pattern called that composes the flagella of a Eukaryotic flagella?
|
9 + 2 pattern.
|
|
|
Flagella function in motility by what type of movement?
|
wave-like movement.
base-to-tip: cell is pushed along tip-to-base: cell is pulled along through a medium |
|
|
Practice: Which statement does NOT correctly summarize a
difference between prokaryotic and eukaryotic cells? a. The electron transport chain of aerobically respiriing prokaryotic and eukaryotic cells is found across the cytoplasmic membrane. b. Whereas prokaryotes generally have a single chromosome, eukaryotes usually have more than 1 chromosome for the storage of genetic material. c. eukaryotic cells have a membrane-bound nucleus whereas prokaryotic cells have only a gel-like mass called the nucleoid. d. Whereas prokaryotic cells secrete enzymes to digest macromolecules, eukaryotes generally bring in large molecules via endocytosis. |
a. The electron transport chain of aerobically respiriing
prokaryotic and eukaryotic cells is found across the cytoplasmic membrane. |
|
|
Which of the following is useful in distinguishing between
prokaryotic and eukaryotic cells? a. the presence / absence of peptidoglycan b. the type of ribosomes used for the synthesis of proteins c. the presence of membrane-delimited organelles within the cytoplasm d. all of the above |
d. all of the above
|
|
|
List the mechanisms of transport
|
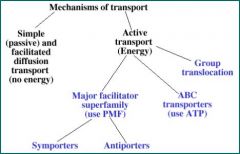
|
|
|
Lateral hairs on a flagellum are called
|
flimmer filaments
|
|
|
Function of Flimmer Filaments:
|
Cause a base-to-tip wave to pull instead of push.
|
|
|
True or False.
Cilia are much shorter than flagella and function in cell motion or may move surrounding material along the cell. |
True.
|
|
|
What are the two phases cilia move:
|
Effective stroke
Recovery stroke |
|
|
Define the effective stroke (cilia movement):
|
Cilium moves through the fluid like an oar pushing the microorganism forward.
|
|
|
Define recovery stroke (cilia movement):
|
cilium bends and repositions itself for the next effective stroke.
|
|
|
True or False.
At any time, some cilia are performing an effective stroke and others are in a recovery stroke. |
True.
|
|
|
Summarize differences between prokaryotic and eukaryotic cells.
|
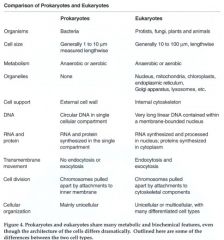
|
|
|
Which two mechanisms of transport require no energy input?
|
Simple (passive) transport
facilitated diffusion transport |
|
|
Which mechanisms of transport require energy?
|
Active transports
|
|
|
Give examples of active transports
|
Group translocation
ABC transporters (use ATP) Major facilitator superfamily (use PMF) > i.e. symporters and antiporters |
|
|
Practice: Transporters using this type of transport require no energy
but become saturated at high substrate concentrations. a. active transport b. simple diffusion c. facilitated diffusion d. none of the above |
c. facilitated diffusion
|
|
|
During what type of transport is a molecule modified as it is
transported? |
Group Translocation
|
|
|
True or False.
Faciliated diffusion is observed much more often in eukaryotic cells than in prcaryotic systems. |
True
|
|
|
Define Active Transport
|
Can accumulate molecules against a concentration gradient (energy required).
|
|
|
Define Major Faciliation Superfamily
|
Transport systems that use the proton motive force (PMF).
|
|
|
Describe two Major Faciliation Superfamilies.
|
Symport
Antiporters |
|
|
Define Symport
|
Symport: linked transport of two substances in the same direction.
One of two Major Faciliation Superfamilies Example: The lactose permease of E. Coli is a symport. |
|
|
Define Antiporters
|
Antiporters: the linked trasnport of two substances in opposite directions.
The sodium pump of E. Coli is an example of such. One of two Major Faciliation Superfamilies |
|
|
Define ABC transport.
|
ATP Binding Cassette
An active transport. Utilizes a binding protein that is located outside the membrane to deliver the molecule to the trasnporter. E. coli uses ABC transporters to bring in a variety of sugars and amino acids. |
|
|
Define Group Translocation
|
A molecule is chemically altered during its passage through the trasnporter (e.g. in the phosphotransferase system, sugars phosphoyrltaed as they enter the cell).
|
|
|
True or False.
The Sec-Dependent Pathway (General Secretion Pathway) is unique to prokaryotes. |
True.
|
|
|
Bacterial cells reproduce via _______
|
binary fission
|
|
|
Describe binary fission
|
a parent cell replicates its DNA,
elongates, a septum forms and eventually cleaves off two distinct daughter cells. With this type of growth, the increase in cell numbers is exponential. |
|
|
Bacterial cell division is classified as what type of growth?
|
exponential.
|
|
|
Practice: If a single cell lands on an agar plate and begins to divide,
how many cells will there be after the tenth cycle of division? |
2^10
|
|
|
Practice: 100 cells of Vibrio fischeri are used to inoculate a large bottle
of sterile media. If this bacteria has a doubling time of 2.5 h, how many cells are present in the culture after an overnight incubation (16 h)? |
100 x 2^6.4 = 8.444 x 10^2
Nt = No X 2n where Nt = the # of cells in a population after a given time No = The original number of cells in a population n = the number of cycles of division (depends on doubling time) |
|
|
Define Microbial growth:
|
An increase in the number of cells in a population.
|
|
|
What kind of system is described below:
nutrients are LIMITED and waste producted are NOT REMOVED. |
Closed (batch) system
|
|
|
Growth in a closed (batch) system are described by?
|
A growth curve.
|
|
|
Practice: During which growth phase do cells begin to synthesize
secondary metabolites in response to increasing cell population and buildup of waste products? a. Lag phase b. Initial log phase c. Late log phase d. Stationary phase e. Death phase |
c. Late log phase
|
|
|
Practice: (T or F) All of the cells in a culture die during the
death phase. |
False.
|
|
|
Practice: Which statement/s regarding the stationary phase of
growth is / are FALSE? a. It is during the stationary phase that culture growth stops. b. During the stationary phase, cells no longer synthesize any metabolites. c. The total number of viable cells stays constant during the stationary phase. d. Secondary metabolites are produced during the stationary phase. e. it is during the stationary phase that cells are most sensitive to antibiotics. f. b and e |
f. b and e
b. During the stationary phase, cells no longer synthesize any metabolites. e. it is during the stationary phase that cells are most sensitive to antibiotics. |
|
|
Define Enzymes.
|
Catalyze reactions by lowering the reaction activation energy.
|
|
|
How do Enzymes lower the reaction activitation energy?
|
1. holding the reactants in such a way that product formation is more
likely. 2. stabilizing the transition state (a transition state is a high-energy complex that has a structure is somewhere between that of products and reactants). |
|
|
Enzymes have an active site at which they bind specifically to their substrate.
Enzymes either fit their substrate precisely are known as? |
lock-and-key
|
|
|
Enzymes have an active site at which they bind specifically to their substrate.
Enzymes that have to change their conformation slightly to fit the substrate are said to be? |
an induced fit.
|
|
|
True or false.
After the reaction has been catalyzed and the products are released, the enzyme remains unchanged. |
True.
|
|
|
Sometimes, two enzymes may recognize the same substrate.
What does this often lead to in a pathway? |
Branching point
|
|
|
Define Allosteric regulation.
|
allosteric regulation is the regulation of an enzyme or other protein by binding an effector molecule at the protein's allosteric site (that is, a site other than the protein's active site). Effectors that enhance the protein's activity are referred to as allosteric activators, whereas those that decrease the protein's activity are called allosteric inhibitors. The term allostery comes from the Greek allos (ἄλλος), "other", and stereos (στερεὀς), "solid (object)", in reference to the fact that the regulatory site of an allosteric protein is physically distinct from its active site. Allosteric regulations are a natural example of control loops, such as feedback from downstream products or feedforward from upstream substrates
|
|
|
During which stage of growth do you think that a bacterium would be most susceptible to antibiotics?
|
Initial Log Phase.
|
|
|
Define Feedback Inhibition
|
The term feedback inhibition refers to a situation in which the substances at the end of a long series of reactions inhibits a reaction at the begining of the series of reactions.
|
|
|
List two toxic derivatives of Oxygen
|
Superoxide
Hydrogen Peroxide *cause damage to cell membranes, DNA and proteins |
|
|
What enzymes detoxify Superoxide? Hydrogen Peroxide?
What will be a visual indicator? |
Superoxide: Superoxide disutase
Hydrogen Peroxide: Catalase Visual indicator: bubbling |
|
|
True or false.
Allosteric bonding is a type of covalent bonding. |
False.
|
|
|
Define Covalent modification
|
the addition or removal of a group (e.g. phosphoryl group)
|
|
|
Define Interconvertible enzymes.
|
Enzymes regulated by covalent modification and modified by converter enzymes.
|
|
|
True or False.
Converter enzymes are often themselves allosterically regulated. |
True.
|
|
|
Define Pacemaker Enzymes.
|
Regulated enzymes which catalyze steps early in a pathway or steps that reuiqre energy input.
|
|
|
What are the benefits of regulating pacemaker enzymes?
|
Reduction of wasteful synthesis or degradation of metabolites.
|
|
|
Define Apoenzyme
|
An enzyme without its cofactor. Inactive.
|
|
|
Define Haloenzyme
|
An enzyme that has its cofactor. Active.
|
|
|
Define Cofactors
|
Cofactors
are always non-protein components and can be split into two groups: essential ions and coenzymes |
|
|
Coenzymes can be divided into which two groups
|
Coenzymes can be further divided into
cosubstrates and prosthetic groups. |
|
|
Define Cosubstrates.
|
cosubstrates can dissociate
from the enzyme (like NADH - that can dissociate and carry it’s electrons to the ETC), |
|
|
Define prosthetic groups:
|
prosthetic groups remain bound to the enzyme (like FADH2 that
can’t ever leave succinate dehydrogenase). |
|
|
Practice: Coenzymes are derivatives of
a. minerals b. proteins c. lipids d. vitamins |
d. vitamins
|
|
|
Practice: For her undergraduate research project a student is
studying a very active enzyme. The student notices that when she treats the enzyme with a solution that removes metal ions, the enzyme suddenly becomes inactive. Explain to the student why this happens. |
Metal ions are acting as cofactors. Removal of the cofactors, makes the enzyme a Apoenzyme.
|
|
|
Define Competitive inhibition
|
an inhibitor (enzyme) actually competes physically with the substrate for access to the active site.
|
|
|
Practice: Which of the following is/are competitive enzyme
inhibitors? a. sulfa drugs b. Magnesium c. NAD+ d. both a and b e. both b and c |
a. sulfa drugs
|
|
|
Define noncompetitive inhibition.
|
the inhibitor and substrate act at different
sites. |
|
|
Competitive and noncompetitive inhibitors can inhibit either reversibly or
irreversibly. Define Irreversible inhibitors. |
Irreversible inhibitors bind covalently and effectively “kill”
the enzyme. Penicillin is an example of an irreversible inhibitor. |
|
|
Competitive and noncompetitive inhibitors can inhibit either reversibly or
irreversibly. Define Reversible inhibitors |
Reversible
inhibitors bind to an enzyme in such a way that the enzyme is unaltered. |
|
|
Define Catabolism
|
Catabolism = Harvesting energy released when a high energy food
molecule is BROKEN DOWN (oxidized, degraded). |
|
|
Define Cofactors.
|
Non-protein components that help enzymes.
|
|
|
What are two groups of Cofactors?
|
Essential Ions
Coenzymes. |
|
|
What are Essential Ions?
|
Metals (commonly Mg and Zn) that may pariticipate in either substrate binding or catalysis.
|
|
|
Coenzymes are often derived from?
|
Vitamins
|
|
|
Coenzymes break down into two groups. Name name.
|
Cosubstrates
Prosthetic Groups |
|
|
Define Cosubstrates.
|
- Altered during the reaction and dissociate from the active site.
- Regain their original structure when acted on by another ezyme - NAD+ and NADP+ |
|
|
What are examples of cosubstrates?
|
NAD+ and NADP+
|
|
|
What is the vitamin precursor of NAD?
|
nicotanimide
|
|
|
Define Prosthetic Groups.
|
Remain bound to the enzyme throught the reaction
Must return to their original form after each catalytic event. Examples: FAD , FMN |
|
|
What are examples of Prosthetic Groups?
|
FAD and FMN
|
|
|
Which vitamin are FAD and FMN derived from?
|
Flavin.
|
|
|
Define Amphibolic
|
mphibolic is used to describe a biochemical pathway that involves both catabolism and anabolism. The citric acid cycle (The Krebs Cycle) is a good example
|
|
|
Define Anabolism
|
Energy (ATP) and reducing power (NADPH) are utilized to
BUILD (synthesize) complex molecules from simple building blocks (e.g. the synthesis of amino acids). |
|
|
Practice: Biosynthetic reactions that require energy for the
conversion of molecular subunits into larger molecules are called a. kinetic energy b. catabolic reactions c. precursor molecules d. anabolic reactions |
d. anabolic reactions
|
|
|
Practice: Concerning catabolism and anabolism
a. they refer to reactions solely dealing with the synthesis of lipids. b. the intermediates of one serve as the reactants in the other. c. the energy gathered in one is utilized in the other. d. they refer solely to the reactions involved in the synthesis of proteins. e. b and c |
e. b and c
b. the intermediates of one serve as the reactants in the other. c. the energy gathered in one is utilized in the other. |
|
|
The First Law of Thermodynamics
|
Energy is never created or destroyed, it is simply converted from
one form to another |
|
|
is it more
favorable for a system (cell) to gain or lose heat? |
Lose heat.
|
|
|
The Second Law of
Thermodynamics |
NATURE SEEKS
CHAOS. The entropy (S) of the universe is always increasing. |
|
|
Is a negative or positive ΔS more favorable?
|
Positiive +
|
|
|
-ΔG is an indicator of what two definitions?
|
spontaneous and exergonic
|
|
|
ΔG+ is an indicator what two definitions?
|
endergonic and nonspontaneous
|
|
|
Practice: Exergonic reactions
a. occur when the value of ΔG is negative. b. are spontaneous c. occur when the value of ΔG is positive. d. both a and b e. both b and c |
d. both a and b
a. occur when the value of ΔG is negative. b. are spontaneous |
|
|
Where does oxidative phosphorylation occur?
|
at the ETC. Synonyms to the PMF
In bacteria the etc is located in the cytosol. In eukaryotes in the inner membrane of mitochondria. |
|
|
Define substrate-level phosphorylation.
|
the phosphorylation of ADP to make ATP
requires energy. If the energy comes from a high-E molecule (e.g. 1,3-BPG) than this is called substrate-level phosphorylation |
|
|
Practice: The name given to the reaction involving removal of
electrons or hydrogen atoms from a compound is termed a. glycolysis b. reduction c. oxidation d. metabolism |
c. oxidation
|
|
|
What does decarboxylation mean?
|
Loss of CO2
|
|
|
Difference between substrate phosphorylation and oxidative phospohorylation.
|
Remember that the phosphorylation of ADP to make ATP
requires energy. If the energy comes from a high-E molecule (e.g. 1,3-BPG) than this is called substrate-level phosphorylation. If, instead, oxidation of NADH / FADH2 forms the PMF and the PMF provides the energy to make ATP, this is called oxidative phosphorylation (OX PHOS). E. Electron carriers - When reduced, NAD+, |
|
|
Define Metabolic Pathways
|
a series of sequential chemical reactions that symphonically regulated to meet the ever-changing neeeds of a cell.
|
|
|
Describe the relationship of photoautotrophs and chemoheterotrophs.
|
Solar energy (kinetic energy) is trapped by photosythenetic microorganisms (PHOTOAUTROPHS) and used to synthesize organic molecules.
In this process, the kinetic light energy is converted to potential energy in the form of bonds. Chemoheterotrophs degrade these organic molecules allowing them to do work (kinetic energy). Photosynthesis >> Cellular Respiration |
|
|
True or False.
Electron carriers are cofactors. |
True.
NAD+, NADP+, FAD |
|
|
Which electron carriers are cofactors?
|
NAD+, NADP+, FAD
|
|
|
How many electrons do NAD+, NADP+, FAD carry?
|
2 e-
|
|
|
Where do NADH and FADH2 carry their electrons to?
|
ETC
|
|
|
Where does NADPH use its electrons?
|
In anabolic (biosynthetic) reactions.
|
|
|
ATP stands for
|
adenosine triphosphate
|
|
|
Practice: The phosphoanhydride bonds of ATP are high in energy because
a. of charge / charge repulsions between the negatively charged oxygen atoms of the phosphoanhydride groups. b. the products of hydrolysis are better solvated than ATP itself. c. the products of hydrolysis are more stable than ATP itself. d. all of the above |
d. all of the above
|
|
|
Where does ATP yield most of its energy from?
|
phosphoester and phosphoanhydride bonds.
|
|
|
Practice: Glycolysis occurs in the ___________ of eukaryotic cells.
a. nucleus b. mitochondria c. cytoplasm d. vacuoles |
Cytoplasm
|
|
|
In which steps is ATP expended in Glycolysis?
|
Steps 1 and 3
|
|
|
Which step is the first commited step of glycolysis?
|
Step 3: the phosphorylation of fructose 6-phosphate, is the first
committed step of glycolysis. This step is catalyzed by phosphofructokinase, an enzyme that can be regulated allosterically by many molecules (two of which are ADP and phosphoenolpyruvate). |
|
|
Practice: Which one of the following statements about
glycolysis is FALSE? a. Glycolysis occurs within the cytoplasm of both prokaryotic and eukaryotic cells. b. During the first step of glycolysis, an ATP molecule is consumed in order to add a phosphate group to glucose. This is a reaction catalyzed by the enzyme hexokinase. c. The fourth step of glycolysis during which the 6- carbon fructose 1,6-bisphosphate molecule is split into two 3-carbon molecules is the committed step in glycolysis. The enzyme that catalyzes this reaction is regulated allosterically by ADP. d. ATP is created for the first time in the seventh step of glycolysis when the high energy phosphate bond in 1,3-bisphosphoglycerate is broken. e. Glycolysis is a amphibolic pathway that occurs in both obligate aerobes and obligate anaerobes. |
c. The fourth step of glycolysis during which the 6-
carbon fructose 1,6-bisphosphate molecule is split into two 3-carbon molecules is the committed step in glycolysis. The enzyme that catalyzes this reaction is regulated allosterically by ADP. |
|
|
True or False.
Glycolysis can occur in either aerobic or anaerobic conditions. |
True.
|
|
|
True or False.
many of the intermediates in glycolysis can serve as precursor metabolites for anabolic pathways |
True.
|
|
|
What is the Pentose Phosphate Pathway?
|
This pathway is an alternate glucose degrading pathway. It is used when
biosynthesis is the primary focus of the cell! |
|
|
What are the functions of the Pentose Phosphate Pathway?
|
The
purpose of the oxidative stage is to produce NADPH. The purpose of the non-oxidative stage is to produce ribose 5-phosphate (used in nucleotide synthesis). Sometimes a cell needs more NADPH than it does ribose 5- phosphate. In such a situations, excess ribose 5-phosphate will be converted to intermediates of glycolysis (fructose 6-phosphate and Glyceraldehyde 3- phosphate). |
|
|
Practice: The pentose phosphate pathway
a. can proceed either in the presence or absence of O2. b. generates NADPH. c. is also termed the hexose monophosphate shunt. d. would be important in a a microorganism trying to synthesize nucleic acids. e. all of the above. |
e. all of the above.
|
|
|
True or False.
The Pentose Phosphate Pathway can operate aerobically or anaerobically. |
True.
|
|
|
Where does the Pentose Phosphate Pathway occur?
|
Occurs in the cytoplasmic matrix of both prokaryotic and eukaryotic cells
|
|
|
True or False.
The Pentose Phosphate Pathway can occur the same time as the Emdben-Meyerhof Pathway? |
True
|
|
|
Embden Meyerhof pathway is also known as?
|
Glycolysis
|
|
|
The Entner-Doudoroff Pathway is restricted to which organisms?
|
Soil Bacteria.
|
|
|
How does the Entner-Doudoroff differ from Glycolysis?
|
The steps of the triose stage are the same to that of Glycolysis (Embden-Meyerhof pathway),
differ in the steps of the hexose stage, which are similiar to thsoe of the pentose phosphate stage. |
|
|
What is the function of fermentation?
|
To regenerate NAD+ for glycolysis to be able to continue, and produce ATP.
|
|
|
Why doesn't the TCA cycle continue in anaerobic conditions?
|
Pyruvate is redirected.
(metabolic pathways are redirected to fermentation). |
|
|
True of False.
Lactic acid fermentation - pyruvic acid itself, serves as the electron acceptor to regenerate NAD+. |
True
|
|
|
Practice: If 45 molecules of glucose are catabolized, how many
NAD+ coenzymes are reduced in the transition step. a. 45 b. 55 c. 90 d. 22.5 |
c. 90
|
|
|
Practice: The transition step between glycolysis and the TCA cycle ...
a. produces two NADH for every molecule of glucose. b. occurs in the same place in prokaryotic and eukaryotic cells. c. releases CO2 as a byproduct. d. both a and b e. both a and c |
e. both a and c
|
|
|
Where does the transition step occur?
|
Prokaryotic cells - cytoplasm
Eukaryotic - mitochondrial matrix |
|
|
True or false.
alpha-ketoglutarate produced in step 3 and oxaloacetate produced in step 8 are important precursor metabolites in the synthesis particular amino acids. |
True
|
|
|
Practice: If 23 molecules of glucose are catabolized, how many
molecules of ATP are produced (via substrate level phosphorylation) by the TCA cycle? How many FADH2 molecules and NADH molecules are produced by the cycle? |
46 FADH2
138 NADH |
|
|
True or False.
the TCA cycle is not used in organisms that lack an electron transport chain or when a terminal electron acceptor is unavailable. |
True.
the TCA cycle does not directly utilize O2 (g), however, it produces a great deal of reducing power (NADH and FADH2) that could not be regenerated without an electron transport chain. |
|
|
Do electrons from NADH or electrons from the oxidation of succinate
(FADH2) generate more energy? |
NADH
|
|
|
Practice: Which electron carrier in the ETC of
mitochondria accepts electrons from NADH and ultimately transfers them to coenzyme Q. a. Complex I b. Cytochrome oxidase c. Complex II d. NADH dehydrogenase e. both b and c f. both a and d |
f. both a and d
|
|
|
Practice: Which member of the mitochondrial ETC is a
lipid soluble molecule that can move freely in the membrane? This electron carrier can accept electrons from either NADH dehydrogenase or Complex II. a. Coenzyme Q b. Cytochrome c c. Ctyochrome c oxidase d. Succinate dehydrogenase |
a. Coenzyme Q
|
|
|
Practice: Shuttling of electrons through which complex/es
of the mitochondrial ETC results in the pumping of protons? a. Complex II b. NADH Dehydrogenase c. Complex III d. Succinate dehydrogenase e. a and b f. b and c |
f. b and c
|
|
|
Define The Chemiosmotic Theory
|
The Chemiosmotic Theory states that the PMF can serve as an
energy source for phosphorylation of ADP to form ATP! |
|
|
True or False.
Anaerobic respiration yields less energy than aerobic respiration. |
True.
|
|
|
Practice: Right now, growing deep in the anaerobic depths of our
Winogradsky columns is a bacterium called Desulfovibrio. This bacterium has the components of the ETC across its cytoplasmic membrane and utilizes sulfur or sulfate as a terminal electron acceptor in a process called _______________. a. anaerobic respiration b. fermentation c. photosynthesis |
a. anaerobic respiration
|
|
|
Practice: A bacterium that can utilize starches as an energy
source must secrete which enzyme/s? a. α-amylase b. oligo 1,6-glucosidase c. cellulases d. lipases and proteases e. a and b f. c and d |
e. a and b
|
|
|
For each pair of electrons delivered to the ETC by NADH, ___ ATP are formed via oxidative phospohorylation.
|
3
|
|
|
For each pair of electrons delivered to the ETC by FADH2/QH2, ___ ATP are formed via oxidative phosphorylation.
|
2
|
|
|
Define Anaerobic respiration
|
Organisms capable of anaerobic respiration can use a molecule other than O2 as the terminal electron acceeptor. Still have ETC.
|
|
|
True or False
Monosaccharides besides glucose can enter the glycolytic pathway. |
True.
|
|
|
Cellulose is digested by what enzymes?
|
cellulases.
|
|
|
Lipids (fats - triglycerides) are hydrolyized by secretory enzyme called:
|
Lipases.
Cleaves the glycerol backbone |
|
|
The glycerol backbone is converted into _____.
|
glyceraldehyde 3-phosphate, an intermediate in glycoysis
|
|
|
Proteins
Which enzymes break the peptide linkages? |
Proteases
|
|
|
True or False.
Chemoautotrophs thrive in extreme environments and use inorganic compounds as an energy source. These compounds are often byproducts of anaerobic respiration (e.g. hydrogen sulfide). |
True.
|
|
|
Practice: The hydrogen bacteria and sulfur bacteria
a. are chemoheterotrophs. b. are chemoautotrophs. c. oxidize hydrogen for energy. d. oxidize hydrogen sulfide for energy. e. b and c |
b. are chemoautotrophs.
|
|
|
True or False.
Prokaryotes are alone in their ability to use inorganic compounds as an energy source. |
True.
|
|
|
Define Photosynthesis
|
the capture and conversion of light energy to chemical
energy. an inorganic carbon source (CO2) is converted to an organic carbon source. |
|
|
True or False.
Prokaryotes are alone in their ability to use inorganic compounds as an energy source. |
True.
|
|
|
Define light-dependent reactions
|
The light-dependent reactions of photosynthesis are the reactions
in which the energy of sunlight is converted into chemical energy in the form of ATP (this is the process happening in photosystems, see below) |
|
|
Define Photosynthesis
|
the capture and conversion of light energy to chemical
energy. an inorganic carbon source (CO2) is converted to an organic carbon source. |
|
|
Define oxygenic photosynthesis:
|
In oxygenic photosynthesis, water provides the source of
electrons. |
|
|
Define light-dependent reactions
|
The light-dependent reactions of photosynthesis are the reactions
in which the energy of sunlight is converted into chemical energy in the form of ATP (this is the process happening in photosystems, see below) |
|
|
Define oxygenic photosynthesis:
|
In oxygenic photosynthesis, water provides the source of
electrons. |
|
|
Define anoxygenic photosynthesis
|
In anoxygenic photosynthesis, a molecule other than
water is the electron source (e.g. H2S). |
|
|
Define anoxygenic photosynthesis
|
In anoxygenic photosynthesis, a molecule other than
water is the electron source (e.g. H2S). |
|
|
Define light-independent reactions (dark reaction)
|
The light-independent reactions of photosynthesis convert CO2
into an organic carbon source (the Calvin Cycle). These reactions don’t directly require light but they require the reducing power and ATP that are products of the light reactions. |
|
|
Define light-independent reactions (dark reaction)
|
The light-independent reactions of photosynthesis convert CO2
into an organic carbon source (the Calvin Cycle). These reactions don’t directly require light but they require the reducing power and ATP that are products of the light reactions. |
|
|
Practice: Although the light-independent (dark) reactions of
photosynthesis do not directly require light, they do require ____________ and ____________________ that are produced during the light reactions. a. oxygen, hydrogen sulfide b. water, carbon dioxide c. ATP, reducing power d. cyanobacteria, purple bacteria |
c. ATP, reducing power
|
|
|
Practice: Although the light-independent (dark) reactions of
photosynthesis do not directly require light, they do require ____________ and ____________________ that are produced during the light reactions. a. oxygen, hydrogen sulfide b. water, carbon dioxide c. ATP, reducing power d. cyanobacteria, purple bacteria |
c. ATP, reducing power
|
|
|
What is the photosynthesis formula?
|
6CO2 + 12 H2X --light---> C6H12O6 + 12X + 6H2O
|
|
|
What is the photosynthesis formula?
|
6CO2 + 12 H2X --light---> C6H12O6 + 12X + 6H2O
|
|
|
Which bacteria would utilize bacteriochlorophyll?
|
purple bacteria
|
|
|
Which bacteria would utilize chlorophyl a and b?
|
Cyanbacteria
|
|
|
Light of various wavelengths are absorbed by?
|
the antenna complex
|
|
|
Light gathering pigments funnel energy toward?
|
the reaction center chlorophyll
|
|
|
Define Photophosphorylation
|
ATP made via light
|
|
|
When a cell needs to make ATP but NOT reducing power,
only photosystem I is used in a process called ________. |
cyclic photophosphorylation.
|
|
|
When a cell needs to make ATP and reducing power, both
photosystem I and II are used in a process called ________. |
noncyclic
photophosphorylation. |
|
|
Practice: During noncyclic photophosphorylation, electrons
are syphoned from the electron transport chain of photosystem I to reduce NADP+. How are these lost electrons replenished? |
PS II
|
|
|
In noncyclic photophosphorylation by oxygenic
phototrophs, the electrons of photosystem I are diverted from the electron transport chain and used to make reducing power in the form of NADPH. These lost electrons are replenished by photosystem II. How does photosystem II replenish its electrons? |
Water
|
|
|
True or False.
The Calvin Cycle, a dark reaction / light independent, is the opposite of the TCA? |
True
|
|
|
In the calvin cycle, how many ATP and NADPH are needed to form one sugar?
|
18 ATP, 12 NADPH
|

