![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
29 Cards in this Set
- Front
- Back
|
Avogadro's Law |
n1 v2 = n2 v1 |
|
|
Ideal Gas Law |
PV= nRT
|
|
|
Combined gas law |
P1 V1 T2= P2 V2 T1 |
|
|
Boyles Gas Law |
P1 V1 = P2 V2 |
|
|
Charles Law |
V1 T2 = V2 T1 |
|
|
Gay-Lucassac Law |
P1 T2= P2 T1 |
|
|
Daltons Law description |
The total pressure of a mixture of gases equals the sum of the partial pressures of the individual gases |
|
|
Daltons Law |
Ptotal= P1 + P2 +....... |
|
|
How many kpa's are in one atm? |
one atm is equal to 101.3 kpa |
|
|
how many mmhg are in one atm? |
one atm is equal to 760 mmhg |
|
|
how many torrs are in one atm? |
760 torrs in one atm |
|
|
What does Boyles law say? In your own words |
boyles law says that if you add more pressure to something then the volume goes down. If you take away pressure from something then the volume goes up. (Ex- Balloon) |
|
|
What does Charles law say in your own words |
if you make the temperature of something go higher then the volume also increases |
|
|
How do you convert degrees Celsius to Kalvin? |
Add 273. To go from Kalvin to celsius you subtract 273 |
|
|
what is STP? |
Standard Temperature and Pressure. Temperature is equal to 0 degrees celcius and pressure is equal to 1 atm |
|
|
Which one of the laws is the inverse law? |
Boyles. Everything else is indirect |
|
|
A sample of gas at 24 degrees Celsius occupies a volume of 3.45 L and exerts a pressure of 2.10 atm the gas is cooled to negative twelve degrees and the pressure is increased to 5.2 atm. Determine the new volume |
Solve. Answer is 1.22 L |
|
|
Sig Fig rules |
Zeroes placed before other digits are not significant
Zeroes placed between other digits are always significant: example: 5008 has 4 significant figures.
Zeroes placed after other digits but behind a decimal point are significant: example: 2.90 has 3 significant figures
Zeroes at the end of a number are significant only if they are behind a decimal point |
|
|
What are the rules to use the ideal gas law? |
Only can use the units of Liters Moles Atmospheres and Kalvin Also the R(or constant) is always 0.0821 Lastly the n is moles |
|
|
Under what conditions of pressure and temperature does a gas behave most idealy? |
Gases are ideal when there is low pressure and high temperature |
|
|
A sample of oxygen is collected over water at 27 degrees Celsius the total pressure is 0.95 atm and the water vapor pressure at 27 degrees Celcius is 26.7 torr. Determine the partial pressure of the oxygen gas collected |
Answer is 695.3 torr OR .915 atm |
|
|
When Calcium Carbonate is heated strongly carbon dioxide gas is evolved; CaCO3-> CaO + CO2 Determine the volume occupied by the carbon dioxide produced by the decompostion of 23.5 g of calcium carbonate. The carbon dioxide is collected at 1.10 atm and 24 degrees celsius. |
answer is |
|
|
what is the SI unit of pressure? Why isnt it used in the USA? |
The Pascal. it isnt used because the number is just too tiny to be practical. |
|
|
synthesis reaction |
A+B --> AB |
|
|
decompostion |
AB--> A+B |
|
|
single displacement |
A+BC --> AC+B |
|
|
double replacement |
AB + CD--> AD + CB |
|
|
combustion |
Ends with CO2 and H2O |
|
|
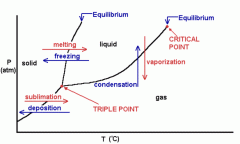
phase diagram |
|

