![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
36 Cards in this Set
- Front
- Back
|
Catalyst |
Speeds up the rate of reaction by lowering the activation energy needed for the reaction Not consumed |
|
|
Ozone |
Form of oxygen containing three atoms in its molecular structure Occurs in upper layers of earth's atmosphere 3O2 + electrolysis = 2O3 More reactive than oxygen Protects harmful UV rays |
|
|
Cold water reactivity |
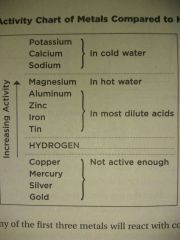
Very active metal + Water = Hydrogen + Metal hydroxide K Ca Na |
|
|
Dilute acids |
Active metal + Dilute acid = Hydrogen + salt of acid Al Zn Fe Sn |
|
|
Preparation of hydrogen |
Electrolysis of water Passing steam over red hot iron or through hot coke Decomposing natural gas with heat CH4+H2O= CO+3H2 |
|
|
Properties of hydrogen |
Colourless,odourless,tasteless when pure Slightly soluble in water Becomes liquid at -240°C and 13 atm Diffuses more rapidly 0.9g/L at 0°C and 1 atm |
|
|
Chemical properties of hydrogen |
Burns in air or oxygen giving off large amounts of heat Does not support combustion Good reducing agent ( withdraws oxygen) |
|
|
Pressure |
Force/Area |
|
|
Atmospheric pressure |
Result of weight of a mixture of gases Approximately 10N |
|
|
Normal atmospheric pressure |
Sea-level air pressure which can support 760mm column of mercury |
|
|
Measurement of air pressure |
Mercury barometer Manometer Top meniscus for mercury Lower meniscus for water |
|
|
I atm = ... Hg mm |
769 mm Hg |
|
|
1 atm = ... Torr |
760 Torr |
|
|
1 atm = ...Pa |
101,325 Pa |
|
|
1 atm = ... Kilopascal |
101.325 kilopascal |
|
|
Calculation of pressure |
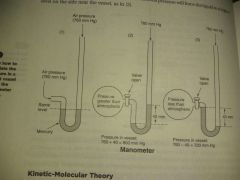
|
|
|
Kinetic molecular theory |
Matter is composed of extremely small particles The space occupied by the gas particles themselves is ingnored in comparison with the whole volume occupied of the container The particles are in constant motion When these particles collide with eachother or the walls of the container there is no loss of energy |
|
|
Temperature |
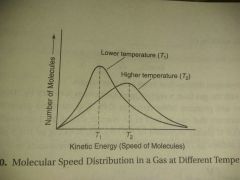
Average kinetic energy |
|
|
Diffusion |
The random motion of gases in moving from one position to another |
|
|
Effusion |
Passing of gas through an oriface into an evacuated chamber. |
|
|
Effusion |
Passing of gas through an oriface into an evacuated chamber. |
|
|
Graham's law of effusion |
The rate of effusion is inversely proportional to the square root of its molecular mass Rate A/Rate B = √molecular mass of B/ √molecular mass of A |
|
|
STP |
Standard temperature - 273K and 0°C Standard pressure - 1 atm |
|
|
Charles law |
V1/T1= V2/T2 At constant pressure |
|
|
Boyle's law |
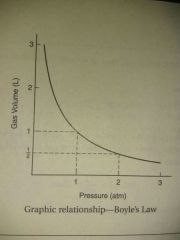
P1V1=P2V2 at constant temperature |
|
|
Combined gas law |
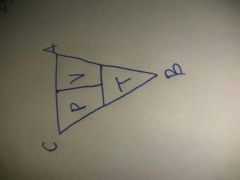
P1V1/T1= P2V2/T2
|
|
|
Gay Lussac's law |
P1/T1=P2/T2 At constant volume |
|
|
Dalton's law of partial pressure |
In a mixture of gases , the total pressure = sum of partial pressures P(total)= P(gas1)+P(gas2)+P(gas3) |
|
|
Partial pressure |
65% of nitrogen,15% of oxygen at 760mm 0.65×760 0.15×760 |
|
|
Ideal gas law |
PV=nRT |
|
|
R |
0.0821 L.atm/mol.K |
|
|
Correction of pressure when level.is higher inside (when mercury) |
Outside reading - barometric pressure (height of mercury) And then correct the partial pressure by subtracting water vapour pressure |
|
|
Correction of pressure ( in case of water) and is higher inside |
Divide the pressure with 13.6 and then subtract from mercury level |
|
|
Correction of pressure when lower inside |
Addition of difference |
|
|
Ideal gas behaviour |
Under conditions of low pressure and high temperature |
|
|
Partial pressure |
Moles of gas/ Total moles in container= partial pressure/ Total pressure |

