![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
12 Cards in this Set
- Front
- Back
|
Standard temperature and pressure (STP) |
Condition where temperature is 273.15K or 0°C and 1 atm 1 atm = 10^5 Pa= 760mmHg = 760 Torr |
|
|
Ideal gas |
Ideal gas represent a hypothetical gas whose molecules have no intermolecular forces and occupy no volume. Under low pressure and high temperature , many gases behave in a nearly ideal fashion. |
|
|
Boyle's law |
Under isothermal condition PV is constant . Pressure and volume are inversely proportional
P1V1 = P2V2
|
|
|
Charles's law |
At constant pressure , V/T is constant V1 / T1 = V2 / T2 T in Kelvin is °C + 273.15 -273.15° C is the lowest attainable temperature known as absolute zero |
|
|
Avogadro's principle |
For all gases at a constant temperature and pressure, the volume of the gas will be directly proportional to the number of moles of gas present
Therefore, all gases have the same number of moles in the same volume
N1 / V1 = N2 / V2
|
|
|
Ideal gas law |
Combine Boyle's law, Charles law , and Avogadro's principle PV = nRT R is the gas constant , under STP, one mole of ideal gas has a volume of 22.4L , R is equal to 8.21X 10-² L*atm / (mol* K ) Or 8.314J/(K*mol) |
|
|
Density |
Mass per unit volume m/ V
|
|
|
Real gases |
Pressure At moderately high pressure , the volume of gas is less than would be predicted by the ideal gas law, this is due to intermolecular attraction
At extremely high pressure , the particle of gas is much bigger compare to the distance between them, so gas take up more volume than would be predicted by the ideal gas law.
Temperature As temperature of gas is decreased , the average velocity of gas molecule decreases, and the intermolecular force become increasingly significant. The closer the temperature of a gas approach boiling point, the less ideal its behavior The more intermolecular force, the less volume it takes up
|
|
|
Dalton's law of partial pressure |
Total pressure of a gaseous mixture is equal to the sum of the partial pressure of the individual components
Ptotal = Pa + Pb + Pc + ...
Pa = Xa Ptotal
|
|
|
Kinetic molecular theory |
Assumptions : 1. Gases are made up of particles whose volumes are negligible compared to the container volume 2. Gas atoms or molecules are inert and exhibit no intermolecular attraction or repulsion 3. Gas particles are in continuous, random motion, undergoing collision with other particles and the container wall 4. Collision between any two gas particles are elastic , meaning that there is no overall gain or lose of energy 5. The average kinetic energy of gas particles is proportional to the absolute temperature of the gas and is the same for all gases at a given temperature. KE = ½mv² = 3/2 kT |
|
|
Maxwell- Boltzmann's distribution curve |
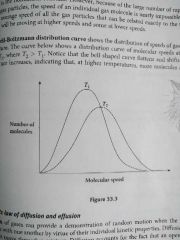
T2 > T1 The bell shaped curve flattens and shifts to the right as the temperature increases, indicate that , at higher temperature, more molecules are moving at high speeds. |
|
|
Graham's law of diffusion and effusion |
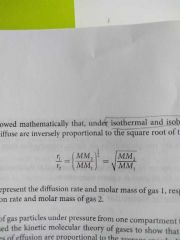
The heavier the gas molecules, the slower it diffuse |

