![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
8 Cards in this Set
- Front
- Back
|
BOYLES LAW Def |
At a constant temperature,the volume of a given mass of gas is inversely proportional to is pressure |
|
|
Gas def |
A substance that has no well defined boundaries but diffuses rapidly to fill any container in which it is placed |
|
|
Charles law def |
At a constant pressure,the volume of a given mass of gas is directly proportional to its temperature measured in kelvin. |
|
|
Avogardros law def |
Equal volume of ideal gases,contain the same number of molecules,under the same conditions. |
|
|
Boyles law formula |
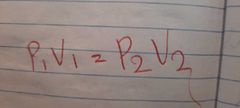
|
|
|
Charles Law formula |
V1/T1=V2/T2 |
|
|
Combined Gas law |
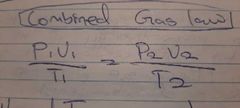
|
|
|
Gay-lussacs's law of combining volume def |

|

