![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
135 Cards in this Set
- Front
- Back
|
Examples of physical preservation factors other than heat? |
1. Irradiation 2. High pressure processing 3. Pulsed electric field 4. UV and Pulsed light |
|
|
Why are alternatives to heat preservation more desirable? |
Heat preservation techniques degrade the quality of food quickly (especially water soluble vitamins). |
|
|
Which parts of the radiation spectrum are usable in food preservation? |
In order of increasing size of wavelength: 1. Gamma rays 2. X-rays 3. Ultraviolet Rays 4. Radar 5. FM/Radio Frequencies |
|
|
Most of effective type of irradiation is based on what chemical property? |
Ionization radiation! *Can release electrons and ionize atoms or molecules |
|
|
What types of irradiations use ionizing radiation? |
1. Electron beam (Beta) 2. Gamma (y) 3. X-rays |
|
|
How does electron beam radiation work? |
Electron beam (beta rays) radiation directly hit surface of food- does not penetrate very far. |
|
|
How does gamma radiation work? |
Gamma rays (radioactive isotopes) emit radiation in all direction from the source, and some of the rays hit the food source. |
|
|
How does X-ray radiation work? |
Electron beam emits X-rays that hit a target plate and the plate in turn emits X-rays to food source. |
|
|
What radioactive isotopes are used for gamma radiation in food applications? |
Cobalt-60 and Caesium-137 |
|
|
What does kGy stand for? What is it a measure of? |
-kilo Grays -Measured radiation dose (not dependent on time) |
|
|
Typical radiation dose relevant to food? |
Range: 0.05 (insects)-30 (viruses) kGy |
|
|
How is radiation lethality measured? |
Radiation D-value in kGy |
|
|
What is the radiation D-value? |
Radiation dose necessary to inactivate 90% (one log) of the population of a given microorganism (in kGy) |
|
|
What types of foods are treated with radiation? |
Foods in packages- this is an advantage Frozen foods- preferable (don't have to thaw/heat), less effective when frozen but still useful |
|
|
What are the mechanisms of action for radiation lethality? |
Reactive products: free-radical generation from water radiolysis (eg OH-) Cellular target: DNA and potentially proteins (or fats). DNA may mutate
|
|
|
Order of radiation resistance |
In order of increasing radiation resistance: 1. Parasites and insects (D-value: 0.1 kGy) 2. Vegetative/bacterial cells (D-value: 0.3-0.7kGy- usually 0.5 used) 3. Bacterial spores (D-value: 2.8 kGy) 4. Viruses (D-value: >10 kGy) |
|
|
Radiation resistance and relevance to genome size |
The larger the genome the easier the organism is to kill |
|
|
If you wanted to pasteurize juice for E. coli with a D-value of 0.4kGy- what would your treatment dose be? |
2 kGy! Rationale: 5 log reduction is considered pasteurized so 0.4x5=2 |
|
|
What application is radiation approved for in wheat flour? |
Control of mold Dose applied: 0.2-0.5kGy |
|
|
What application is radiation approved for in white potatoes? |
Inhibition of sprouting Dose applied: 0.05-0.15kGy |
|
|
What application is radiation approved for in pork? |
Kill parasites Dose applied: 0.3-1.0 kGy |
|
|
What application is radiation approved for in fruit and vegetables? |
Insect control and increased shelf life Dose applied: 1.0 kGy |
|
|
What application is radiation approved for in herbs and spices? |
Sterilization (can only use gamma radiation to do this) Dose applied: 30 kGy |
|
|
What application is radiation approved for in poultry? |
Bacterial pathogen reduction Dose applied: 3kGy |
|
|
What application is radiation approved for in meat (general)? |
Bacterial pathogen reduction Dose applied: 4.5kGy |
|
|
True or false: irradiation causes food to be radioactive for a short period of time. |
FALSE |
|
|
FDA considers radiation as what? |
An additive- radiation treated food has "radura" logo |
|
|
What is UHP? |
Ultra High Pressure processing- food is subjected to high hydrostatic pressure (>300 Mpa) for a relatively short time (minutes). *May keep more nutrients |
|
|
Why is UHP is considered a non-thermal process? |
Does not generate excessive heat. Pressure acts instantaneously and uniform throughout the product, regardless of size/shape/food composition Static pressure |
|
|
UHP food is considered pasteurized or sterilized? |
It is effective against non-spore forming pathogens, and often considered "pasteurized" |
|
|
What does MPa stand for? |
Mega Pascal! Pressure applied to food usually between 300-900 MPa |
|
|
What are the processing parameters necessary for UHP? |
Pressure in MPa (300-900MPa) Holding time (less than 10 minutes) Holding temperature (4C to 120C)
Temperature must always be mentioned |
|
|
What foods are treated with UHP? |
-Packaged foods (with at least one flexible side) - solids or liquids -batch mode |
|
|
What is Le Chatelier's principle? |
"A system at equilibrium tends to minimize the effect of an external perturbation" In english: After applying pressure system tries to compensate for it when its at equilibrium- so it will favor reaction direction with least resistance. *Lethality depends on what reaction is favored at that pressure. * Can cause protein denaturation
|
|
|
What is molecular ordering in regards to UHP? |
-At a constant temperature, an increase in pressure increases the degree of ordering of the molecules of a substance. -Protein unfolding/denaturing depends on water reordering |
|
|
How can you tell how the M/O is responding to a treatment? |
M/O always responds to outside stimulus (may not be a large response though). -May trigger gene expression and protein translation -Can collect mRNA and do DNA microarray to see which gene is expressed after stimulus |
|
|
Which genes respond to UHP? |
DNA microarray data points to Fe-S cluster assembly genes being down-regulated. --> so proteins containing these clusters are sensitive to high pressure (causes release of Fe or S) Example: Fe-S clusters found in respiration and phosphorylation pathway in M/O- so after UHP proteins will lose hydrogens and cannot complete electron transfer chain |
|
|
How does UHP kill bacteria? |
Protein denaturation: iron-sulfur containing proteins (MOST!), electrostatic and hydrophobic interactions (embedded in membrane so can be destroyed and decreases membrane fluidity) Membrane destruction (physics): decreased fluidity (from destruction of hydrophobic components in membrane), and increased permeability Ribosome dissociation DNA replication- can stop this from occurring |
|
|
M/o relative resistance to pressure? |
In order of decreased resistance: 1. Spores are very resistant 2. Gram Positive 3. Gram Negative (more sensitive) 4. Viruses (very sensitive)
|
|
|
Factors affecting M/O resistance to UHP |
-Temperature: generally high temp increases lethality but defeats purpose if too much (ie its destructive to food) -pH: low pH (acidic!) enhances pressure lethality -aw: low aw decreases lethality (as always) |
|
|
What is PEF? |
Pulsed electric field preservation. - Food is subjected to intense electric pulses (>20 kV/cm) for a very short duration (micro to milliseconds) "non thermal process" |
|
|
What type of food is suitable for PEF and why |
Liquid foods having low electric conductivity (like fruit juices)- don't want food to spark. -Effective against non-spore forming pathogens, with the goal of pasteurizing the food |
|
|
PEF processing parameters |
Electric field strength: 20-50 kV/cm Treatment time: 100-1000 micro seconds |
|
|
Foods treated by PEF? |
-Liquid products -Low conductivity *treated in a continuous mode |
|
|
How does PEF inactive M/O? |
Basically by causing pores to form in cell membrane. -If minor defects (ie not enough kV/cm applied) the cell can reseal pores. -If cell becomes permeabilized it will start leaking cell material -If critical defects occur (excessive holes!)- cell dies. Critical EF for lethality ~12kV/cm |
|
|
M/O relative resistance to PEF |
In order of increased sensitivity: 1. Viruses are super resistant 2. Spores 3. Gram positive 4. Gram negative
|
|
|
Whats more effective? Gamma radiation or PEF? WHY? |
Gamma radiation! It causes damage to DNA whereas PEF only effects the cell membrane |
|
|
Factors affecting resistance to PEF |
Temperature: higher temps increase lethality pH: low pH enhances lethality aw: low aw decreases lethality |
|
|
Examples of applications for PEF? |
Food: fruit juices, extractions of intracellular components (like starch from potatoes) Non-food: medicine delivery (trans-dermal pulse delivery, cancer treatments), waste disposal- Applying PEF to solid waste causes water loss and increased liquid extraction which is easier to treat |
|
|
Differences in D-value for thermal and non-thermal processes? |
Thermal D-value: minutes of holding at a given temperature to achieve a 90% reduction Irradiation D-value: radiation dose (kGy) to achieve a 90% reduction (no time) HPP D-value: Minutes of holding at a given pressure (MPa) to achieve a 90% reduction PEF D-value: Micro seconds-milliseconds of PEF treatment at a given electric field strength (kV/cm) to achieve a 90% reduction |
|
|
Inorganic salts that can be used as AMAs? |
Nitrites and NaCl (but usually at too high of a concentration) |
|
|
Acids used as AMAs |
Inorganic: phosphoric acid, HCl Organic: Acetic, Propionic, lactic.. Lipophilic (organic) acids: benzoic, sorbic acid Esters: Parabens |
|
|
Biopreservatives used as AMAs |
Bacteriocins: nisin and pediocin |
|
|
Aw modifiers used as AMAs |
Humectants that bind water! Sugar alcohols: sorbitol Sucrose fatty acid esters |
|
|
Antioxidants used as AMAs |
Ascorbic acid, BHA, BHT |
|
|
Food colorant that can be an AMA |
Red #3? |
|
|
Nitrites and nitrates use as AMAs- background in foods and M/O inhibition |
Only added if M/O present will reduce them (Enterobacteriaceae) -Inhibit Clostridium species --> prevents botulism! -Clostridium botulinum -Other clostridia: C. tyrobutyricum, C. sporogenes, C. perfringens Foods: processed meats/hot dogs/sliced meats/sausages.. some dairy. Restricted to 130-150ppm max |
|
|
Reaction of nitrites in food to stabilize meat color |
Nitrite (NaNO2)--> Nitrous acid HNO2--> Nitric oxide (NO) Nitric Oxide (NO) + Myoglobin--> Nitrosomyoglobin (bright red meat) *Reacts with the Fe center of the myoglobin |
|
|
Nitrites inhibition of anaerobic spore-formers |
1. If M/O contains Ferredoxin or hydrogenase enzymes to reduce pyruvate- then sensitive to nitrites. 2. Fe-S cluster proteins are sensitive to nitrites (so ferredoxin and hydrogenase) ** Reason why LAB don't care about nitrites: they don't have Ferredoxin |
|
|
Cancer causing compound that can be formed by nitrites |
Nitrosamine- formed under high heat (grilling temperatures) in the presence of secondary amines in meats |
|
|
Lipophilic acids properties as AMA agents |
1. The longer the R group, the increased lipophilic nature so less soluble in water. 2. Want free acids in foods but won't mix- so use salts. |
|
|
Dissociated vs undissociated forms of lipophilic acid AMAs |
Undissociated forms have a higher AMA activity than the salt form (dissociated). *Play with pH to get free acid |
|
|
General concept of Henderson-Hasselbach equation and AMA activity. |
Equation: pH= pKa + log (R-COO-)/(R-COOH) *If pH is higher than pKa= AMA activity not favored and vice versa. |
|
|
Lipophilic acids as AMA mechanisms of action |
Absorption: depends on pH of medium (lower pH more absorption) pH gradient across membrane: disruption by lipophilic acids (protonation of compound) Leakage of protons: causes cell energy loss and eventual death In English: Lipophilic acids can easily get across lipid-bound cell membrane, which causes a drop in pH in the cytoplasm, cell tries to compensate by hydrogen pumping out of cell but that requires ATP- so eventually the cell uses up resources and dies |
|
|
Lipophilic acids effectiveness and pH |
Lipophilic acids are added in their salt forms: K or Na salts (dissociated forms) -Undissociated form is the active form- produced if food is acidic. -Extent of dissociation depends on the pKa and pH of the food. |
|
|
Sorbic Acid as an AMA |
Looks like a fatty acid chain but with two double bonds. -pKa: 4.8 Foods: acidic foods: bread, cheese (0.3%/w), yogurts, dried fruits, gelatin, jelly, syrup, sauces, soft drinks, wine (300ppm) ** can't be used in mold ripened cheeses because it inhibits them too. Main uses: primarily inhibits molds and yeasts. Works against S. aureus but not LAB. Safety: body metabolized it |
|
|
Benzoic Acid as an AMA |
pKa: 4.2 Naturally occurring in cranberries, prunes, plums, cinnamon, ripe cloves, most berries. Foods: acidic foods, at a max level of 0.1% Spectrum: molds and yeasts, bacteria, and fungistatic and fungicidal agent. |
|
|
Parabens as AMAs |
Benzoic acid substitutes -esters of p-hydroxybenzoic acid pKa: 8.47
|
|
|
Define Biocontrol |
Control of a pest (pathogen) using biological agents (living organisms or products of living organisms)
-Can be viewed as "more natural approach": exclusion of chemical preservatives -Can be viewed with skepticism by uneducated consumers: viruses in my food? |
|
|
Strategies of biocontrol include? |
Bacteriophages, Bacteriocins- AMA peptides produced by some M/O Competitive exclusion: find M/O that compete with M/O of concern (ie Samonella vs Citrobacter) |
|
|
What is a bacteriophage? |
Viruses that only infect bacteria (usually specific to a strain of bacteria). Extremely ubiquitous Myoviridae= non enveloped, dsDNA |
|
|
What are the three modes of action of a bacteriophage? |
Lysogenic cycle: DNA is intergrated into host genome (prophage/temperate phage), DNA is replicated when cells reproduce, Can be responsible for acquired virulence factors
Lytic cycle: phage takes control of host machinery for reproduction, numerous phages are assembled within the cell, eventually leading to lysis and release of phage particles "lysis from within"
"lysis from without": Many phages attack one cell- cell collapses from multiple attacks. Most convenient method as you can add lots of phage and it will attack even if the cell isn't metabolically active |
|
|
Favorable arguments for bacteriophages |
*Highly specific- ability to target microbe of concern without affecting microbiome *No activity in absence of host microorganism *No health risk to humans and animals *May offer alternative to antibiotic treatments |
|
|
Arguments against the use of bacteriophages |
*Efficacy can be limited- lack of stability in acidic environments *May require optimum host growth conditions for activity- high temperature, high initial population as target *Making contact can be a problem |
|
|
Current uses of bacteriophages |
*Phage therapy: administration of phage preparation to fight disease, historic precedent, especially in Russia/Eastern Europe --Resurgence may be an Rx later *Food contact surfaces *Antimicrobial additives: Listex P100- GRAS processing aid -ListShield: 99-100% reduction -Ecoshield: approval pending -Salmonella products in development |
|
|
What are bacteriocins? |
*Bioactive peptides produced by bacteria: producer is immune to bacteriocin produced *Membrane active compounds: disruption of proton motive force (energy production), disruption of pH gradient, leakage of small molecules (ATP leaves) *More active against Gram positive organisms (ie E. coli is g- and has lots of layers so not effective) *More often associated with LAB |
|
|
What is Nisin? |
-Bacteriocin produced by Lactococcus lactis strains Approved for use in foods (FAO 1969, USDFA- 1988). Effective against Gram positive: Lactic acid bacteria (Lactococcus, Lactobacillus, Streptococcus), Pathogens (Staphylococcus, Listeria), Sporostatic (Clostridium, Bacillus) |
|
|
Arguments for the use of bacteriocins |
*Stability- resistant to heat and low pH *Safety- denaturation by proteolytic enzymes (in body) *Commercial production is possible |
|
|
Arguments against the use of bacteriocins |
*Can be difficult to produce commercially- medium, aeration (LAB anaerobic and microaerotolerant) *Not soluble at neutral/basic pH: not suitable for use above pH~5 *Not active against Gram negative pathogens *Targeting- not specific |
|
|
What is competitive exclusion? |
Use of live microorganisms to displace pathogens! -Competition for nutrients -Competition for binding sites (intestinal epithelium) -Secretion of AMAs: acids, bacteriocins, toxic metabolites
*Biopreservation: fermented foods
LAB competitive exclusion study from ~18yrs ago: inoculated milk with LAB to prevent L. monocytogenes, except L. monocytogenes grows similarly to LAB so it wasn't effective. |
|
|
What are probiotics? |
Definition: live microorganisms which when administered in adequate amounts confer a health benefit on the host.
Presumed health benefits: maintaining and restoring digestive balance, promotion of digestive health and increased immunity |
|
|
What are prebiotics? |
Food ingredients not digested by humans that stimulate growth/activity of probiotics (ex. inulin) |
|
|
What are synbiotics? |
Food products containing both prebiotics and probiotics. |
|
|
What does the label claim "Foods with live active cultures" mean? |
Any microbes added to food and remain live in the product after manufacturing |
|
|
Characteristics of probiotics |
-Non-pathogenic/human origins -Tolerant of acid and bile -Survival in anaerobic environment -Able to adhere to mucosal surfaces (of the intestine) -Provision of "health benefits" -Survival in delivery vehicles |
|
|
Common cultures used as probiotics |
LAB -history of use in fermented foods -Survive in food products -safe for consumption -Produce acid (good for GI tract) -GRAS status |
|
|
Genera often used as probiotics? |
Lactobacillus spp. (LAB) Bifidobacterium spp. (non-LAB) |
|
|
Mechanisms of action of probiotics |
Competitive exclusion -Adhesion to epithelial surface -Competition for nutrients with pathogens or M/O that cause GI issues
Production of antiomicrobials -Acid, hydrogen peroxide, volatile fatty acids -bacteriocins
Enhanced immune response -Bacterial antigens |
|
|
Methods for efficacy testing of probiotics |
Cultural methods -incubation of cells in acidified media/gastric juice
In vitro testing- attachment studies -Cell culture using Caco-2 (SI epithelium cells) or HT-29 (colon epithelium) cell lines
In vivo animal testing -Human testing (ideal for probiotics) -Placebo control a must **BUT- its expensive, time consuming, specific to one microorganism, one delivery method, one target condition, lack of adequate testing leads to uncertainty |
|
|
Probiotic strains for prevention of antibiotic associated diarrhea |
Saccharomyces boulardii Lactobacillus rhanmosus GG |
|
|
Probiotic strain for the prevention of Clostridium difficile disease |
Saccharomyces boulardii |
|
|
Other Digestive claimed health benefits or general benefits of probiotics? |
*Reduced duration of rotavirus diarrhea in children *Reduction in symptoms of lactose intolerance *Reduced risk of colon cancer *Reduction of cholesterol in hyperlipidemic adults |
|
|
Probiotic strain for the prevention of bad breath |
Streptococcus salivarius |
|
|
Probiotic strain for prevention of infant colic |
Lactobacillus reuteri DSM 17938 |
|
|
Considerations for effective dosage of probiotics |
-Estimated 10^9-10^10 viable cells must be consumed daily -Population reduction in the stomach (aggressive HCl) -Short residence time (lack of colonization?)- needs to be taken every day -Viability loss during storage |
|
|
Heat killed probiotic for reduction of rotavirus diarrhea (in humans) |
Lactobacillus acidophilus |
|
|
Heat killed probiotic for the increased eradication of Helicobacter pylori (in humans) |
Lactobacillus acidophilus |
|
|
Heat killed probiotic for the reduced rate of Salmonella infection (murine) |
Lactobacillus acidophilus |
|
|
Heat killed probiotic for the suppression of allergic response (murine) |
Lactobacillus plantarum L-137 |
|
|
Adverse effects of probiotics and M/O known to cause them |
Flatulence -Heterofermentative LAB: Lactobacillus reuteri, Lactobacillus casei, Lactobacillus plantarum
Diarrhea
Serious consequences -Sepsis in immuno-compromised individuals (many probiotics are opportunistic pathogens) -documented with bacterial and fungal probiotics -Induction of widespread inflammatory response --> ulcerative colitis, documented in cases of enteral administration in large doses
|
|
|
Examples of commercially available probiotic foods |
Infant formulas -Lactobacillus rhamnosis, Bifidobacterium lactis
Probiotic yogurt -Lactobacillus acidophilus, Lactobacillus bulgaricus
Fruit drinks Chocolate bars Supplements -available as capsules or chewables, pure cultures or mixtures, 3-50 billion CFU/capsules
Personal Care products soap, tooth paste, baby lotion |
|
|
What is the focus of cleaning and sanitization? |
Focus- food factory equipment For safety and good keeping quality |
|
|
What does cleaning mean for food factory equipment? |
A process of removing visible soils- which is done to prevent accumulation of food residues that may provide right conditions to pathogenic or spoilage microorganisms to grow |
|
|
What does sanitization mean for food factory equipment? |
Treatment of cleaned surfaces by chemical agents (or heat) in order to reduce the population of M/O which are threats for public health, without unfavorably affecting the foods safety and quality.
Population reduction expected? |
|
|
"Cleaned and sanitized surfaces" must meet what conditions in regards to bacterial populations? |
Bacterial populations should: -Not exceed 1 cfu/cm^2 on the food contact surfaces, as measured by swab test -Not exceed 1cfu/ml in the rinsing water, when the rinse test is performed -Be free of coliform microorganisms |
|
|
List common cleaning agents |
Water -good cleaning agent if combined with enough external energy (heat, force)
Surfactants (aka detergents) -Reduce water surface tension -Increased water ability to wet surfaces, lift contaminates by mechanical action, and carry away soils
Acids or alkalis -Vinegar vs baking soda -Dissolving power of minerals, fats, proteins
Chelating agents -Water softening -Calcium has divalent cations so add something to remove Enzymes |
|
|
Water solubility of soil ingredients |
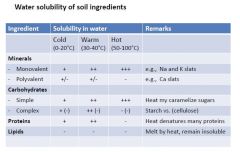
|
|
|
Types of cleaning chemicals |
Surfactants Have a hydrophobic tail and a hydrophilic head The head can be non-ionic (long/no charge), anionic (-) or cationic (+)
Anionic -Soap (long fatty acid chain) -Sodium dodecyl sulfate (also has long fatty acid chain)
Non-anionic: -Long chain alcohols -Triton X-100 |
|
|
Emulsification by detergents |
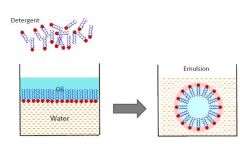
|
|
|
Types of cleaning chemicals- acids & bases |
*ACIDS Single or a mixture of acids, with or without wetting agents -pH of acid detergent is often 2.5 or less -Great solubilizing power -Can be corrosive
Inorganic -Sulfuric, nitric, phosphoric etc
Organic -Citric, acetic, tartaric, etc.
*BASES/ALKALI -often containing sodium hydroxide (caustic detergents) -Others include trisodium phosphate, sodium metasilicate -sodium pH is well in the basic range -Excellent saponifying effect -Can be corrosive |
|
|
Characteristics of cleaners (chemical) |
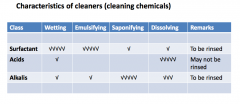
|
|
|
How are detergents applied? |
Manual -Disassemble the equipment -Rinse with cold or warm water -Brush with detergent solution -Rinse with water -Assemble the equipment |
|
|
What is CIP? |
Cleaning-in-place technology
-Complete cleaning of equipment without disassembling or any kind of manual intervention.
-Done by circulation or flowing, by mechanical means through a piping system, of a detergent solution, water rinse,and sanitizing solution. |
|
|
Sanitization and sanitizers |
Definition: applying antimicrobial chemicals (sanitizers) to decontaminate food-contact surfaces that has been adequately cleaned.
Sanitizers -inactivate vegetative cells of M/O of public health significance -substantially decrease the viability of other undesirable microorganisms -Does not adversely affect the quality of the product -Does not adversely affect the safety of the consumer -Note: approved sanitizers in the US are those that do not require a rinse after the sanitization step Examples: household bleach, quaternary ammonium compounds |
|
|
Chlorine- Cleaning and sanitization |
Chlorine gas Sodium hypochlorite (solution): Production: NaOH+O2--> NaOCl+NaCl+ H20
-Advantages: easy to produce, stable at storage, detergent effect because of high pH (11 to 12)
-Disadvantages: low sanitizing effect at high pH, safety concerns about trihalomethanes, off odor to some foods
AMA modes of action: -NaOCl+H+ --> HOCl+ Na+ HOCl----> H(+)+ Cl(-) +O(nascent, reactive, germicide)
Potency: Concentration/time pH Temperature Presence of organic material Resistance of some M/O
Forms liquid bleach calcium hypochlorite (solid) Chlorinated trisodium phosphate (solid)
Uses Sanitize equipment: 50-200ppm |
|
|
Quaternary ammounium compounds (Quats) |
Serves as a sanitizer and cationic wetting agent. Structure -R groups vary -Permanently charged, regardless of pH Examples: benzalkonium chloride, benzethonium chloride Effectiveness Act by disrupting the cell membrane Effective against vegetative bacteria, fungi, amoeba, and enveloped viruses Advantages Stable, toxicity and corrosiveness, temperature stable, pH Disadvantages Expensive, lack of efficacy against spores, and non-enveloped viruses, foaming, off-flavors |
|
|
Assessments of M/O quality of food |
Quantitative (Enumeration) -Major microbiotic (eg mesophilic aerobic count) -Yeasts and mold count -Enterobacteriaceae (or coliform) count
|
|
|
Assessment of food safety |
Qualitative (Detection) -Detection of pathogens of concern or toxins in a food
Eg: detection of Escherichia coli O157:H7 in raw meat |
|
|
Techniques vs methods |
Sample: a small and manageable quantity intended to represent the whole
Method: a collection of techniques, when executed produces south information. Ex: Genetic based detection of Listeria monocytogenes in food. *Includes techniques in this particular method: Enrichment, Selection, Polymerase chain reaction (PRC)
Techniques: A collection of analytical steps. Ex: PCR techniques in the above method include the following steps: cell lysis, dna extraction, adding reagents, amplification by thermocycling, band separation by gel electrophoresis.
|
|
|
Microbiological Analytical Techniques- Microscopic |
Light: cell and colony morphology, cell counting Electron: sub cellular components |
|
|
Microbiological Analytical Techniques- Culture |
Isolation, Enumeration, Enrichment |
|
|
Microbiological Analytical Techniques-Biochemical |
Identification (ie Catalyst, Nitrate reduction..) Biotyping |
|
|
Microbiological Analytical Techniques-Immunological |
Enzyme immunoassays (ELIZA) Immuno-fluorescence Serotyping (antigen labeling) |
|
|
Microbiological Analytical Techniques-Genetic |
DNA-DNA hybridization- randomly-amplified polymorphic DNA Polymerase chain reactions: Pulsed field gel electropheresis Genetic typing- Ribotyping **typing refers to subspecies level |
|
|
Basics of pathogen detection |
~ usually takes 3 days to a week -Sampling -Sample preparation -Pathogen amplification -Selection and screening -Identification -Pathogenicity testing |
|
|
Laboratory sample vs Analytical sample |
Laboratory sample -Thawing, if the sample was delivered frozen -Partitioning, shredding, or grinding of solid samples -Thorough mixing (solids or liquids)
Analytical sample (subsample) -Measuring -Homogenization -Dilution (if necessary |
|
|
Pathogen amplification considerations |
-When present, pathogens found in small amounts -Finding a pathogen of concern in a food matrix is often difficult -Need to amplify the pathogen to detectable levels Problem: other unwanted M/O are also amplified. Therefore, selection/screening is needed.
Amplification techniques: enrichment, PCR |
|
|
Pathogen detection in foods |
Example: Listeria monocytogenes in ground meat detection -Major microbiota: 10^6 cfu/g -Listeria: 1 cfu/g
*Considering a bacteria cell dimensions, a cell weights ~10^-12g (picograms) Therefore detection level- 1ppt |
|
|
Selective enrichment of Listeria in Fraser broth |
Selective agents: will not effect Listeria but will inhibit other M/O examples: nalidixic acid, acriflavin, lithium chloride (inhibtis enterococci)
Differential agents: will effect Listeria differently than other similar M/Os (ie color change) examples; Esculin, ferric ammonium citrate (FAC) *medium turns black in the presence of Listeria |
|
|
Identification |
Determining (or confirming) the identity of an isolate by matching its characteristics with those of a previously identified species.
-Characteristics: morphological, biochemical, physiological, genetic
*Start broad (ie gram staining) and become more specific *ID leads to determining isolate's genus or species *May need to identigy subspecies and infrasubspecies (strains) levels, ie "typing"
Example of typing: hemolysis under high CO2 (Listeria is Beta-hemolytic, so true hemolysis) |
|
|
Pathogenicity testing |
-Cell culture: cytopathogenicity -Animal model: or a tissue in the animal -No testing on humans (obvi): but use to be done on prisoners |
|
|
Method sensitivity |
Sensitivity: proportion of positive test results obtained when the method is applied to samples known to carry the M/O targeted by the analysis
False negative= 100-sensitivity Minimum detection limits: higher sensitivity methods detects smaller numbers
*higher sensitivity= better for use in foods *higher sensitivity= higher accuracy |
|
|
Method specificity |
Specificity: method's ability to distinguish a targeted microorganism from other microorganisms in the sample
False-positive rate= 100-specificity
*Higher specificity= higher precision of method (agreement of set of results among themselves) |
|
|
What is HACCP? |
Hazard Analysis and Critical Control Point (managing the hazard)
CCP: any point in a food processing operation where loss of control can lead to a hazard
Hazard: any biological (M/O), chemical (ex: melamine), or physical property that causes consumer health risks
--> food safety assurance |
|
|
Developing and implementing a HACCP plan (steps) |
1. Assess hazards and risks: find out what problems the specific food in question can cause 2. Determine the critical control points: typical CCP are: heat treatments, refrigeration/freezing step, steps where pH is lowered or maintained, food handling and employee hygiene. 3. Establishing critical limits: Example= pasteurization of milk: 71.7C for 15S. Minimum is the set temperature/time. Maximum is set at the point where the product becomes unacceptable. 4. Establish procedures to monitor CCPs: equipment to monitor, when to monitor/reprocess 5. Corrective measures: what to do when things go wrong.. reprocess? throw away? 6. Record keeping: tracking problems.. law suits. 7. Verification: Is it really working? |

