![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
45 Cards in this Set
- Front
- Back
|
In addition to reactive groups from amino acid side chains, enzymes may contain non-protein molecules. Give two examples.
|
A prosthetic group, and a coenzyme.
|
|
|
Coenzymes are small organic molecules that can be loosely or tightly bound to an enzyme. Tightly bound coenzymes can be called...
|
Allosteric groups
|
|
|
What is an 'allosteric group'?
|
Coenzymes are small organic molecules that can be loosely or tightly bound to an enzyme. Tightly bound coenzymes can be called allosteric groups.
|
|
|
What is a prosthetic group? (wrt enzymes)
|
A *covalently* bonded organic compound or chelated (Forms a compound containing a ligand bonded to a central metal atom at two or more points with) metal ion
|
|
|
What does 'chelated' mean?
|
Form a chelate with
--> A compound containing a ligand (typically organic) bonded to a central metal atom at two or more points |
|
|
'A *covalently* bonded organic compound or chelated (Forms a compound containing a ligand bonded to a central metal atom at two or more points with) metal ion'
|
A prosthetic group
|
|
|
Prosthetic groups are covalently bonded to enzymes T/F
|
T (coenzymes are not)
|
|
|
'Tightly, but *not* covalently, bound organic compound that can be removed from an enzyme (e.g. by dialysis)'.
|
Coenzyme
|
|
|
What is a coenzyme?
|
Tightly, but *not* covalently, bound organic compound that can be removed from an enzyme (e.g. by dialysis).
|
|
|
Coeznymes are covalently bonded to enzymes T/F
|
F (prosthetic groups are)
|
|
|
Coenzymes can be removed from enzymes T/F If so, how?
|
T --> Dialysis
|
|
|
What is dialysis?
|
The separation of particles in a liquid on the basis of differences in their ability to pass through a membrane
|
|
|
'The separation of particles in a liquid on the basis of differences in their ability to pass through a membrane'
|
Dialysis
|
|
|
Enzyme + prosthetic group/coenzyme =
|
Holo-enzyme (catalytically active)
|
|
|
What is a holo-enzyme (wrt 'equation')?
|
Enzyme + prosthetic group (a holo-enzyme is catalytically active)
|
|
|
A holo-enzyme is catalytically inactive T/F
|
F (An apo-enzyme is catalytically inactive)
|
|
|
A holo-enzyme is catalyticall active T/F
|
T
2120160211 - On train, just left Marylebone after formative mocks |
|
|
'An enzyme without a prosthetic group or coenzyme'
|
An apo-enzyme (catalytically inactive)
|
|
|
What is an 'apo-enzyme'?
|
An enzyme without a prosthetic group or coenzyme. It is catalytically inactive.
|
|
|
The active site of an enzyme can be considered to consist of:
|
A binding site and a catalytic site
|
|
|
Which part of the active site of an enzyme is being described?
'Non-covalent interactions between amino acid side-chains and the substrate(s)' |
The binding site
|
|
|
You need to learn the acidic/basic/hydrophilic/aromatic etc. amino acids.
|
They are in the lecture notes
|
|
|
Does multiple interactions with the active site lead to a high, or low degree of specificity for the substrate(s) bound?
|
High
|
|
|
Enzymes can/cannot distinguish between D- and L-isomers and between cis- and trans-isomers
|
Can
|
|
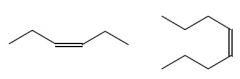
Cis/Trans?
|
Cis
|
|
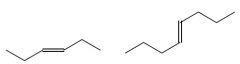
Cis/trans?
|
Trans
|
|
|
Reactive groups on the surface of the enzyme catalyse the reaction by:
Donating or withdrawing electrons T/F |
T
|
|
|
Reactive groups on the surface of the enzyme catalyse the reaction by:
Stabilising or generating free radical intermediates T/F |
T
|
|
|
Reactive groups on the surface of the enzyme catalyse the reaction by:
Forming temporary covalent bonds T/F |
T
|
|
|
Enzymes act by increasing the activateion energy of the reaction T/F
|
F, they lower the activation energy
|
|
|
Enzymes act by increasing the speed at which equilibrium is achieved T/F
|
T
|
|
|
Enzymes act by altering the position of the equilibrium T/F
|
F
|
|
|
What are the six groups of enzymes?
|
Oxidorenductases
Transferases Hydrolases Lyases Isomerases Ligases (synthetases) |
|
|
Need to learn about enzyme classification
|
In lecture notes - systematic number (EC number)
e.g. EC 4.1.1.28 (understand this) |
|
|
Hydrolysis of esters of basic amino acids
|
Trypsin
|
|
|
Hydrolysis of esters of aromatic amino acids
|
Chymotrypsin
|
|
|
Hydrolysis of esters of small neutral amino acids
|
Elastase
|
|
|
What is the change to the primary AA sequence to cause the varient form of methylene tetrahydrofolate reductase (MTHFR)
|
Alanine 226 mutated to valine (leads to abnormal metabolism of folic acid, can be overcome with high intakes of folate)
Same gene is associated with spina bifida and other neural tube defects |
|
|
What is V(max)?
|
The maximum rate of reaction of the enzyme when the enzyme is saturated with substrate
|
|
|
The maximum rate of reaction of the enzyme when the enzyme is saturated with substrate
|
V(max)
|
|
|
What is K(m)?
|
The inverse measure of the affinity of the enzyme for its substrate - it is the concentration of the substrate required to achieve half (V)max
|
|
|
The inverse measure of the affinity of the enzyme for its substrate - it is the concentration of the substrate required to achieve half (V)max
|
K(m)
|
|
|
What are the units of V(max)?
|
mol of product formed/unit time
|
|
|
What are the units of K(m)
|
mol/L (concentration)
|
|

|
Important to understand!
|

