![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
9 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Chemists perform reactions at constant pressure and volume changes are possible, to simplify equation ∆U, what is definition of enthalpy? |
H= U + pV ∆H=∆U + p∆V + V∆p Under isobaric condition ∆p =0 Therefore ∆H=∆U+p∆V |
∆U=q(in)-p∆V |
|
|
What is the substituted formula ∆U=q(in)-p∆V, |
∆H = q(in) |
|
|
|
What is word definition of enthalpy? |
Energy change in a system taking into account that system can do mechanical work. |
|
|
|
What is the pressure of 1 mole of an ideal gas at 298K? |
p=nRT/V p=2477.572 J/V |
|
|
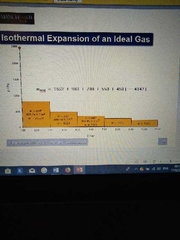
Given the graph, what is the work done when considering isothermal expansion from V¹ to V²? (See hint) |
Need to integrate w(on)= p∆V
w(on)=-nRT ln(V²/V¹) |
internal pressure is affected by external pressure, the volume and pressure will change to balance the pressure. Work done by expanding gas is w(on)= p∆V |
|
|
Why is reversible expansion a theoretical construct? |
The expansion of the gas may only be described as reversible if the entire expansion occurs in infinitesimally small increment. For an expansion to take place purely by infinitesimal increment requires infinite time. |
|
|
|
Why is reversible expansion useful? |
Maximum expansion work is obtained when the external pressure is only infinitesimally smaller than the internal pressure at all stages of expansion. |
|
|
|
In a process where heat is applied to a system that releases energy as work, for an ideal gas any energy supplied as heat must leave as work: q(in)= -w(on) What is the formula for q(in) if expansion is also reversible? |
q(in)=nRT ln(V²/V¹) |
|
|
|
What is change in entropy? |
It is reversible heat of a system devided by temperature. ∆S=nR ln(V²/V¹) Expanding gas has positive change in entropy |
|

