![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
60 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Covalent bonds |
Strong molecular interactions mediated by shared electrons |
|
|
|
Non covalent bonds |
Weak reversible molecular interactions |
|
|
|
Types of non covalent bonds |
Ionic bonds Hydrogen bonds Vander waals bonds |
|
|
|
Ionic bonds |
Mediated by oppposite electrostatic charges |
|
|
|
Hydrogen bonds |
Mediated by a shared hydrogen atom |
|
|
|
Van der waals bonds |
A nonspecific attraction (occurs when any two atoms are 3-4 A• apart |
|
|
|
Water |
Polar Triangular Highly cohesive Excellent solvent for polar molecules Weakens ionic and H- bonds |
|
|
|
Water |
Polar Triangular Highly cohesive Excellent solvent for polar molecules Weakens ionic and H- bonds |
|
|

Carbonic anhydrase |
Catalyzes the reaction between CO2 and H2O Extremely fast enzyme Located largely in erythrocytes and kidneys A metalloenzyme contains zinc |
|
|
|
Most CO2 is transported in the blood as |
Bicarbonate |
|
|
|
Laws of thermodynamics |
First law: the total energy of a closed system is conserved Second law: the entropy of a closed system always increases |
|
|
|
Laws of thermodynamics |
First law: the total energy of a closed system is conserved Second law: the entropy of a closed system always increases |
|
|
|
Enthalpy is the heat content of a system |
Entropy is the degree of disorder of a system |
|
|
|
Direct calorimetry |
Direct measurement of the amount of heat produced in a given system |
|
|
|
Direct calorimetry |
Direct measurement of the amount of heat produced in a given system |
|
|
|
Indirect calorimetry |
Measurement of the amount of heat produced in terms of inhaled O2 and exhaled CO2 |
|
|
|
Enzymes |
Highly specific catalysts for biochemical reactions Classified according to their mechanism of action |
|
|
|
Examples of metallic coenzymes of various enzymes Copper- cytochrome oxidase Iron- catalase Peroxidase |
Magnesium- hexokinase , glucose-6- phosphatase Pyruvate kinase Nickel- urease Zinc- carbonic anhydrase, Alcohol dehydrogenase |
|
|
|
Enzymes composed of proteins combined with non protein structures (either organic or inorganic) that aid in their function |
Coenzymes Cofactors Prosthetic groups |
|
|
|
Coenzyme |
Non protein portion of an enzyme |
|
|
|
Apoenzyme |
Protein portion of an enzyme Catalytically inactive by itself |
|
|
|
Halo enzyme |
Complete catalytically active enzyme Apoenzyme + coenzyme |
|
|
|
Isoenzymes |
Enzymes with subtle molecular differences that catalyze the same reaction |
|
|
|
Classification of enzymes OVER THE HILL |
Oxidoreductases Transferases Hydrolases Isomerases Lyases Ligases |
|
|
|
Oxidoreductases |
Catalyze redox reactions |
|
|
|
Transferases |
Catalyze the transfer of functional groups |
|
|
|
Transferases |
Catalyze the transfer of functional groups |
|
|
|
Hydrolases |
Catalyze bond cleavage by hydrolysis |
|
|
|
Transferases |
Catalyze the transfer of functional groups |
|
|
|
Hydrolases |
Catalyze bond cleavage by hydrolysis |
|
|
|
Isomerases |
Catalyze a change in molecular structure |
|
|
|
Transferases |
Catalyze the transfer of functional groups |
|
|
|
Hydrolases |
Catalyze bond cleavage by hydrolysis |
|
|
|
Isomerases |
Catalyze a change in molecular structure |
|
|
|
Lyases |
Catalyze bond cleavage by elimination |
|
|
|
Ligases |
Catalyze the union of two molecules |
|
|
|
Metals and B complex vitamins |
Serve as the majority of nonprotein coenzymes |
|
|
|
Metals and B complex vitamins |
Serve as the majority of nonprotein coenzymes |
|
|
|
Induced fit model: Substrate binding induces a conformational change in an enzyme |
The energy produced by these changes enables the reactions to progress |
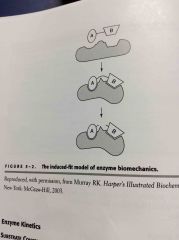
|
|
|
Enzyme kinetics Substrate concentration: Increasing substrate concentration increases reaction rate only until the enzyme binding sites are saturated |
Maximum reaction velocity (Vmax) is achieved when any further increase in substrate concentration does not increase reaction rate |
|
|
|
Enzyme kinetics Substrate concentration: Increasing substrate concentration increases reaction rate only until the enzyme binding sites are saturated |
Maximum reaction velocity (Vmax) is achieved when any further increase in substrate concentration does not increase reaction rate |
|
|
|
The Michaelis constant (Km) is the |
Substrate concentration when the initial reaction velocity is half of the maximum reaction velocity Illustrated mathematically by the Michaelis- Menten equation |
|
|
|
Km reflects the affinity of the enzyme for its substrate Km is indirectly proportional to enzyme affinity |
Vmax is directly proportional to the substrate concentration |
|
|
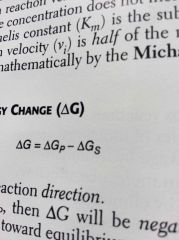
Gibbs free energy change |
Determines reaction direction If delta Gs > delta Gp, then delta G will be negative and the reaction will proceed spontaneously toward equilibrium Equilibrium is attained when delta G=0 |
|
|
|
Michaelis Menten equation |
Vi = Vmax . [S]/ Km + [S] |
|
|
|
Delta G provides no information about the reaction rate and is independent of the path of the reaction |
Reactions are based on their delta G Exergonic Endergonic |
|
|
|
Reaction direction |
Determined by the delta G |
|
|
|
Reaction equilibrium A. A + B + Enz = C + D + Enz B. Keq = [C][D][Enz]/ [A][B][Enz] |
A: any reaction with enzyme present B: equilibrium constant of the reaction Enzymes have no effect on reaction equilibrium |
|
|
|
Activation energy |
Is the energy needed to initiate a reaction |
|
|
|
Reaction rate : Determined by the activation energy Attaining activation energy requires an increase in reactant kinetic energy |
Kinetic energy is largely influenced by temperature and substrate concentration Enzymes lower the activation energy of a reaction accelerating the rate |
|
|
|
What are the factors influencing reaction rate |
pH Temperature Enzyme concentration Inhibitor concentration Substrate concentration |
|
|
|
Classification of reactions based on delta G: Reaction type: exergonic Delta G: negative Energy flow: released |
Reaction type: endergonic Delta G : positive Energy flow: required |
|
|
|
Change in factor for pH: Extreme changes can alter the charged state of the enzyme or substrate |
Characteristics of pH: Enzymes function within an optimal pH range |
|
|
|
Change in factor for pH: Extreme changes can alter the charged state of the enzyme or substrate |
Characteristics of pH: Enzymes function within an optimal pH range |
|
|
|
Change in factor for temperature: An increase in temperature will increase the reaction rate |
Characteristics of temperature: Extreme increase in temperature can cause enzyme denaturation |
|
|
|
Change in factor for pH: Extreme changes can alter the charged state of the enzyme or substrate |
Characteristics of pH: Enzymes function within an optimal pH range |
|
|
|
Change in factor for temperature: An increase in temperature will increase the reaction rate |
Characteristics of temperature: Extreme increase in temperature can cause enzyme denaturation |
|
|
|
Change in factor for enzyme concentration |
An increase in enzyme concentration will increase the reaction rate |
|
|
|
Change in factor for inhibitor concentration |
An increase in inhibitor concentration will decrease the reaction rate |
|
|
|
Change in factor for substrate concentration: An increase in substrate concentration will increase reaction rate only until the enzyme binding sites are saturated |
Characteristics of substrate concentration: Enzymes have a limited number of active sites |
|

