![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
24 Cards in this Set
- Front
- Back
|
What is the normal range of blood pH in mammals?
|
7.4
<7.35 - acidemia >7.45 alkalemia |
|
|
What happens to the CNS if there is acidemia and alkalemia?
|
Acidemia - depression, coma
Alkalemia - hyperactivity, tetany of respiratory muscle |
|
|
Is the dihydrogen phosphate or the monohydrogen phosphate the acid form of phosphate?
|
Dihydrogen
|
|
|
Name 4 buffers of pH in the blood
|
Bicarbonate
Phosphate Proteins Histidines (in RBCs) |
|
|
How do buffers work?
|
They either release or accept protons
|
|
|
How does a buffer operate if the blood is acidic?
|
Accepts proton
|
|
|
What is the main difference between carbonic and lactic acid?
|
Carbonic is volatile
Lactic is not |
|
|
Which parts of the body deal with non-volatile and volatile acids?
|
Lungs - volatile
Kidneys - non-volatile |
|
|
How does the body maintain the pH of 7.4 in relation to bicarbonate and carbonic acid?
|
Depends on the ratio of bicarbonate to carbonic acid
|
|
|
What is the relation of pCO2 and pH?
|
Can work out concentration of carbonic acid in blood to work out pH
|
|
|
How is bicarbonate a perfect buffer in the lungs?
|
Carbon dioxide produced in body makes blood too acidic, some of this carbonic acid is "blown off" in the lungs and increases the buffer to 7.4
|
|
|
When is bicarbonate not a perfect buffer?
|
This happens because the lungs cannot change the bicarbonate in the blood, only the carbonic acid component. So if bicarbonate is lowered in blood bla bla
|
|
|
Why can bicarbonate buffer far from its pK of 6.1?
|
Because the volatile component of carbonic acid, CO2, can be blown off in the lungs
|
|
|
Where in the nephron is bicarbonate absorbed?
|
In the proximal tubule
|
|
|
Describe the reabsorption of bicarbonate in the proximal tubule
|
Bicarbonate passes freely through glomerulus
Bicarbonate is protonated by H ion from Na/H antiporter on epithelial cell to H2CO3 Carbonic acid is dehydrated to CO2 and H2O by luminal carbonic anhydrase Carbon dioxide diffuses into cell CO2 and H2O hydrated to bicarbonate + proton by cytoplasmic carbonic anhydrase (isozyme) |
|
|
What enables the Na/H antiporter to provide proton to bicarbonate?
|
Sodium potassium pump - creates low sodium in cell
|
|
|
How does the kidney produce bicarbonate into the blood?
|
CO2 used to create bicarbonate + proton
Bicarbonate released into blood stream Proton added to phosphate to dihydrogen phosphate which is used to buffer the urine |
|
|
What enables the phosphate buffering?
|
Na K pump
|
|
|
What happens to the dihydrogen phosphate formed?
|
Either taken into blood
Or excreted in urine |
|
|
What occurs when non-volatile acid production is too high for the kidney to cope with?
|
Instead of phosphate buffering ammonia is used
|
|
|
Where does the ammonia arise from?
|
Glutamine - amino acid - dissociating into glutamate and ammonia
|
|
|
How does ammonia buffer the urine?
|
Ammonia binds with the excreted proton that could not bind to phosphate
Also bicarbonate released into blood |
|
|
DRIVEN BY
|
SODIUM POTASSIUM PUMP
|
|
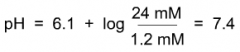
Which line of the fraction is affected by which organ?
|
Top line - kidney - non-volatile acids
Bottom line - lungs - CO2 |

