![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
133 Cards in this Set
- Front
- Back
|
What is the mechanism of Heparin? |
Cofactor for the activation of antithrombin, ↓ thrombin, and ↓ factor Xa |
|
|
What are the uses of Heparin? |
- Immediate anticoagulation for Pulmonary Embolism, Acute Coronary Syndrome, MI, and Deep Venous Thrombosis |
|
|
What do you need to monitor in patients taking Heparin? |
Follow PTT |
|
|
What are the toxic side effects of Heparin? |
- Bleeding |
|
|
What is HIT? |
Heparin Induced Thrombocytopenia (HIT) |
|
|
How do you rapidly reverse an overdose of Heparin? Mechanism? |
Protamine Sulfate (positively charged molecule that binds negatively charged heparin) |
|
|
What are the types of low molecular weight heparins (LMWH)? Mechanism? How are they different? |
Enoxaparin and Dalteparin |
|
|
What drugs can you use in a patient who requires anticoagulation but was taking heparin and got heparin-induced thrombocytopenia (HIT)? Source? |
Argatroban or Bivalirudin |
|
|
What is the mechanism and use of Argatroban? |
- Inhibits thrombin directly |
|
|
What is the mechanism and use of Bivalirudin? |
- Inhibits thrombin directly |
|
|
What is the mechanism of Warfarin? |
Interferes with normal synthesis and γ-carboxylation of vitamin K-dependent clotting factors II, VII, IX, and X and proteins C and S |
|
|
What clotting factors and proteins are affected by Warfarin? |
- Factor II |
|
|
What is the effect of warfarin on blood tests? |
Increases PT (extrinsic pathway) / INR |
|
|
What are the clinical uses of Warfarin? |
- Chronic anti-coagulation (after STEMI, venous thromboembolism prophylaxis, and prevention of stroke in atrial fibrillation) |
|
|
What are the toxic side effects of Warfarin? |

- Bleeding |
|
|
How can you reverse a warfarin overdose? |
- Give Vitamin K |
|
|
What drugs are direct factor Xa inhibitors? |
- Apixaban |
|
|
What is the mechanism of Apixaban and Rivaroxaban? |
Binds and directly inhibits activity of factor Xa |
|
|
What are the clinical uses of Apixaban and Rivaroxaban? |
- Treatment and prophylaxis of DVT and PE (rivaroxaban) |
|
|
What are the side effects of Apixaban and Rivaroxaban? |
Bleeding (no specific reversal agent available) |
|
|
What is the structure of heparin and warfarin? |
- Heparin: large, anionic, acidic polymer |
|
|
What is the route of administration of heparin and warfarin? |
- Heparin: parenteral (IV, SC) |
|
|
What is the site of action of heparin and warfarin? |
- Heparin: blood |
|
|
What is the relative onset of action of heparin and warfarin? |
- Heparin: rapid (seconds) |
|
|
What is the mechanism of action of heparin and warfarin? |
- Heparin: activates antithrombin, which ↓ the action of IIa (thrombin) and factor Xa |
|
|
What is the duration of action of heparin and warfarin? |
- Heparin: acute (hours) |
|
|
Do heparin and warfarin inhibit coagulation in vitro? |
- Heparin: yes |
|
|
How do you treat an acute overdose of heparin and warfarin? |
- Heparin: protamine sulfate |
|
|
What should you monitor in patients taking heparin and warfarin? |
- Heparin: PTT (intrinsic pathway) |
|
|
Do heparin and warfarin cross the placenta? |
- Heparin: no |
|
|
What are the types of thrombolytics? |
- Alteplase (tPA) |
|
|
What is the mechanism of Alteplase (tPA), Reteplase (rPA), and Tenecteplase (TNK-tPA)? |
Thrombolytics |
|
|
What are the clinical uses of Alteplase (tPA), Reteplase (rPA), and Tenecteplase (TNK-tPA)? |
- Early MI |
|
|
What are the side effects and contraindications of Alteplase (tPA), Reteplase (rPA), and Tenecteplase (TNK-tPA)? |
- Bleeding |
|
|
How do you treat toxicity of Alteplase (tPA), Reteplase (rPA), and Tenecteplase (TNK-tPA)? Mechanism? |
- Aminocaproic Acid - inhibits fibrinolysis |
|
|
What is the mechanism of aspirin (ASA)? |
- Irreversibly inhibits cyclooxygenase (both COX-1 and COX-2) enzyme by covalent acetylation |
|
|
What lab values change in patients taking aspirin (ASA)? |
- ↑ Bleeding time, ↓ TXA2 and prostaglandins |
|
|
What are the clinical uses of aspirin (ASA)? |
- Antipyretic |
|
|
What are the toxic side effects of aspirin (ASA)? |
- Gastric ulceration |
|
|
What can an overdose of aspirin (ASA) cause? |
Respiratory alkalosis initially, which is then superimposed by metabolic acidosis |
|
|
What is the cause of Reye syndrome? |
Children with a viral infection who take aspirin |
|
|
What are the types of ADP receptor inhibitors? |
- Clopidogrel |
|
|
What is the mechanism of Clopidogrel, Ticlopidine, Prasugrel, and Ticagrelor? |
- Inhibit platelet aggregation by irreversibly blocking ADP receptors |
|
|
What are the clinical uses of Clopidogrel, Ticlopidine, Prasugrel, and Ticagrelor? |
- Acute coronary syndrome |
|
|
What are the side effects of Clopidogrel, Ticlopidine, Prasugrel, and Ticagrelor? |
- Neutropenia (ticlopidine) |
|
|
What drugs inhibit platelet aggregation via inhibition of phosphodiesterase III? |
Cilostazol and Dipyridamole |
|
|
What is the mechanism of Cilostazol and Dipyridamole? |
- Phosphodiesterase III inhibitor |
|
|
What are the uses of Cilostazol and Dipyridamole? |
- Intermittent claudication |
|
|
What are the side effects of Cilostazol and Dipyridamole? |
- Nausea |
|
|
What are the types of GpIIb/IIIa inhibitors? |
- Abciximab |
|
|
What is the mechanism of Abciximab, Eptifibatide, and Tirofiban? |
- Binds to the glycoprotein receptor IIb/IIIa on activated platelets, preventing aggregation |
|
|
What are the uses of Abciximab, Eptifibatide, and Tirofiban (GpIIb/IIIa inhibitors)? |
- Unstable angina |
|
|
What are the side effects of Abciximab, Eptifibatide, and Tirofiban (GpIIb/IIIa inhibitors)? |
- Bleeding |
|
|
What are the drugs that interact with the cell cycle? Which part? |
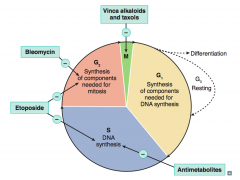
- Antimetabolites (affect S) |
|
|
What anti-neoplastic drugs affect nucleotide synthesis? How? |
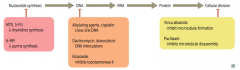
- Methotrexate and 5-Fluorouracil: ↓ thymidine synthesis |
|
|
What anti-neoplastic drugs inhibit DNA synthesis? How? |
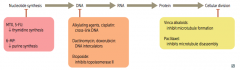
- Alkylating agents, Cisplatin: cross-link DNA |
|
|
What anti-neoplastic drugs inhibit cellular division? How? |
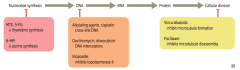
- Vinca alkaloids: inhibit microtubule formation |
|
|
What are the anti-metabolite drugs? |
- Methotrexate (MTX) |
|
|
What is the mechanism of Methotrexate (MTX)? |
Folic acid analog that inhibits Dihydrofolate Reductase → ↓ dTMP → ↓ DNA and ↓ protein synthesis |
|
|
What are the clinical uses of Methotrexate (MTX)? |
- Cancers: leukemias, lymphomas, choriocarcinoma, sarcomas |
|
|
What are the toxic side effects of Methotrexate (MTX)? |
- Myelosuppression |
|
|
How can you reverse the myelosuppression induced by Methotrexate (MTX)? |
Reversible with leucovorin (folinic acid) "rescue" |
|
|
What is the mechanism of 5-Flurouracil (5-FU)? |
- Pyrimidine analog bioactivated to 5F-dUMP, which covalently complexes folic acid |
|
|
What is 5-fluorouracil (5-FU) used for? |
- Colon cancer |
|
|
What are the toxic side effects of 5-fluorouracil (5-FU)? |
- Myelosuppression |
|
|
How can you reverse the myelosuppression induced by 5-Fluorouracil (5-FU)? |
Reversible with uridine "rescue" |
|
|
What is the mechanism of Cytarabine (Arabinofuranosyl Cytidine)? |
Pyrimidine analog → inhibition of DNA polymerase |
|
|
What is Cytarabine (Arabinofuranosyl Cytidine) used for? |
- Leukemias |
|
|
What are the toxic side effects of Cytarabine (Arabinofuranosyl Cytidine)? |
- Leukopenia |
|
|
What is the mechanism of Azathioprine, 6-Mercaptopurine (6-MP), and 6-Thioguanine (6-TG)? |
- Purine (thiol) analogs → ↓ de novo purine synthesis |
|
|
What are the clinical uses of Azathioprine, 6-Mercaptopurine (6-MP), and 6-Thioguanine (6-TG)? |
- Preventing organ rejection |
|
|
What are the toxic side effects of Azathioprine, 6-Mercaptopurine (6-MP), and 6-Thioguanine (6-TG)? |
- Bone marrow, GI, and liver toxicity |
|
|
What are the anti-tumor antibiotics? |
- Dactinomycin (Actinomycin D) |
|
|
What is the mechanism of Dactinomycin (Actinomycin D)? |
Intercalates in DNA |
|
|
What are the clinical uses of Dactinomycin (Actinomycin D)? |
- Wilms tumor |
|
|
What are the toxic side effects of Dactinomycin (Actinomycin D)? |
Myelosuppression |
|
|
What is the mechanism of Doxorubicin (Adriamycin) and Daunorubicin? |
- Generates free radicals |
|
|
What are the clinical uses of Doxorubicin (Adriamycin) and Daunorubicin? |
- Solid tumors |
|
|
What are the toxic side effects of Doxorubicin (Adriamycin) and Daunorubicin? |
- Cardiotoxicity (dilated cardiomyopathy) |
|
|
What is the mechanism of Bleomycin? |
Induces free radical formation, which causes breaks in DNA strands |
|
|
What are the clinical uses of Bleomycin? |
- Testicular cancer |
|
|
What are the toxic side effects of Bleomycin? |
- Pulmonary fibrosis |
|
|
What are the alkylating agent drugs? |
- Cyclophosphamide, Ifosfamide |
|
|
What is the mechanism of Cyclophosphamide and Ifosfamide? |
- Covalently X-link (interstrand) |
|
|
What are the clinical uses of Cyclophosphamide and Ifosfamide? |
- Solid tumors |
|
|
What are the toxic side effects of Cyclophosphamide and Ifosfamide? |
- Myelosuppression |
|
|
How can you limit the hemorrhagic cystitis caused by Cyclophosphamide and Ifosfamide? Mechanism? |
Mesna - thiol group of mesna binds toxic metabolites |
|
|
What is the mechanism of Nitrosoureas (Carmustine, Lomustine, Semustine, and Streptozocin)? |
- Requires bioactivation |
|
|
What are the clinical uses of Nitrosoureas (Carmustine, Lomustine, Semustine, and Streptozocin)? |
Brain tumors (including glioblastoma multiforme) |
|
|
What are the toxic side effects of Nitrosoureas (Carmustine, Lomustine, Semustine, and Streptozocin)? |
CNS toxicity (convulsions, dizziness, ataxia) |
|
|
What is the mechanism of Busulfan? |
Cross-links DNA |
|
|
What are the clinical uses of Busulfan? |
- CML |
|
|
What are the toxic side effects of Nitrosoureas (Carmustine, Lomustine, Semustine, and Streptozocin)? |
- Severe myelosuppression (in almost all cases) |
|
|
What are the types of microtubule inhibitors? |
- Vincristine and Vinblastine |
|
|
What is the mechanism of Vincristine and Vinblastine? |
- Vinca alkaloids that bind β-tubulin |
|
|
What are the clinical uses of Vincristine and Vinblastine? |
- Solid tumors |
|
|
What are the toxic side effects of Vincristine and Vinblastine? |
- Vincristine: neurotoxicity (areflexia, peripheral neuritis), paralytic ileus |
|
|
What is the mechanism of Paclitaxel and other Taxols? |
- Hyperstabilize polymerized microtubules in M phase so that mitotic spindle cannot break down (anaphase cannot occur) |
|
|
What are the clinical uses of Paclitaxel and other Taxols? |
Ovarian and breast carcinomas |
|
|
What are the toxic side effects of Paclitaxel and other Taxols? |
- Myelosuppression |
|
|
What is the mechanism and clinical uses of Cisplatin and Carboplatin? |
- Cross-links DNA |
|
|
What are the toxic side effects of Cisplatin and Carboplatin? |
- Nephrotoxicity |
|
|
What is the mechanism and clinical uses of Etoposide and Teniposide? |
- Inhibits topoisomerase II → ↑ DNA degradation |
|
|
What are the toxic side effects of Etoposide and Teniposide? |
- Myelosuppression |
|
|
What is the mechanism and clinical uses of Irinotecan and Topotecan? |
- Inhibits topoisomerase I and prevents DNA unwinding and replication |
|
|
What are the toxic side effects of Irinotecan and Topotecan? |
- Severe myelosuppression |
|
|
What is the mechanism and clinical uses of Hydroxyurea? |
- Inhibits ribonucleotide reductase → ↓ DNA synthesis (S-phase specific) |
|
|
What are the toxic side effects of Hydroxyurea? |
- Bone marrow suppression |
|
|
What is the mechanism of Prednisone and Prednisolone? |
- May trigger apoptosis |
|
|
What are the clinical uses of Prednisone and Prednisolone? |
- Most commonly used glucocorticoids in cancer chemotherapy |
|
|
What are the toxic side effects of Prednisone and Prednisolone? |
Cushing like symptoms: |
|
|
What is the mechanism of Tamoxifen and Raloxifene? |
- Selective estrogen receptor modulator (SERMs) |
|
|
What are the clinical uses of Tamoxifen and Raloxifene? |
- Breast cancer treatment (tamoxifen only) and prevention |
|
|
What are the toxic side effects of Tamoxifen and Raloxifene? |
- Tamoxifen: partial agonist in endometrium, which ↑ the risk of endometrial cancer, "hot flashes" |
|
|
What is the mechanism of Trastuzumab (Herceptin)? |
- Monoclonal antibody against HER-2 (c-erbB2), a tyrosine kinase receptor |
|
|
What are the clinical uses of Trastuzumab (Herceptin)? |
HER-2 positive breast cancer and gastric cancer (Tras2zumab) |
|
|
What are the toxic side effects of Trastuzumab (Herceptin)? |
- Cardiotoxicity ("HEART"ceptin damages the heart) |
|
|
What is the mechanism of Imatinib (Gleevec)? |
Tyrosine kinase inhibitor of bcr-abl (Philadelphia chromosome fusion gene in CML) and c-Kit (common in GI stromal tumors) |
|
|
What are the clinical uses of Imatinib (Gleevec)? |
- CML |
|
|
What are the toxic side effects of Imatinib (Gleevec)? |
Fluid retention |
|
|
What is the mechanism of Rituximab? |
Monoclonal Ab against CD20, which is found on most B-cell neoplasms |
|
|
What are the clinical uses of Rituximab? |
- Non-Hodgkin Lymphoma (CD20 positive cells) |
|
|
What are the toxic side effects of Rituximab? |
Increased risk of progressive multifocal leukoencephalopathy |
|
|
What is the mechanism and use of Vemurafenib? |
- Small molecule inhibitor of forms of the B-Raf kinase with the V600E mutation |
|
|
What is the mechanism and clinical uses of Bevacizumab? |
- Monoclonal antibody against VEGF, inhibits angiogenesis |
|
|
What are the toxic side effects of Bevacizumab? |
Hemorrhage and impaired wound healing |
|
|
Which chemo drug(s) cause(s) acoustic nerve damage? |
Cisplatin / Carboplatin |
|
|
Which chemo drug(s) cause(s) peripheral neuropathy? |
Vincristine |
|
|
Which chemo drug(s) cause(s) pulmonary fibrosis? |
- Bleomycin |
|
|
Which chemo drug(s) cause(s) cardiotoxicity? |
- Doxorubicin |
|
|
Which chemo drug(s) cause(s) nephrotoxicity? |
Cisplatin and Carbolatin (and acoustic nerve damage) |
|
|
Which chemo drug(s) cause(s) hemorrhagic cystitis? |
Cyclophosphamide |
|
|
Which chemo drug(s) cause(s) myelosuppression? |
- 5-FU |

