![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
23 Cards in this Set
- Front
- Back
- 3rd side (hint)
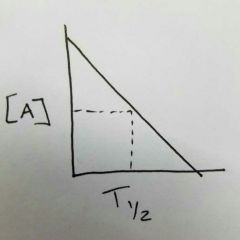
What order is this? What happens to half-life, Rate, and the Rate constant as time progresses? |
Zero order Rate does not change Rate constant does not change |
|
|
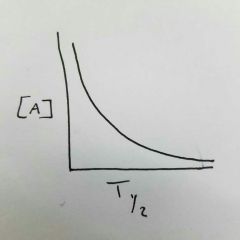
What order is this? What happens to the half-life, rate, rate constant as time progresses? |
First order half-life does not change rate decreases rate constant does not change |
|
|
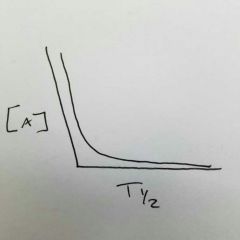
What order is this? What happens to half-life, rate, and rate constant as time progresses? |
Second order half-life increases rate decreases rate constant does not change |
|
|
|
An increase in temperature does what to rate and the rate constant. |
increases the rate of reaction increase rate constant |
|
|
|
Adding a catalyst does what the activation energy and the rate of reaction. |
decreases activation energy increases the rate of reaction |
|
|
|
increasing temperature of liquid does what to the solubility of gases and solids in that liquid? |
decreases solubility of gases increases the solubility of solids |
|
|
|
In a zero order reaction, what happens to the concentration of the reactants? |
does not change because the rate does not change. |
|
|
|
Mass% formula |
Mass % =mass of solute / mass of solution |
|
|
|
Mass% formula |
Mass % =mass of solute / mass of solution |
|
|
|
Mole Fraction formula |
Mole fraction = mole solute / mole solution |
|
|
|
Molality formula |
Molality = moles solute / kg solvent |
|
|
|
Molarity formula |
Molarity = moles solute / Liters solution |
|
|
|
Vapor Pressure depends on... |
1. Temperature 2. Surface area of the liquid 3. Strength of intermolecular forces that hold the molecules together in the liquid |
3 factors |
|
|
Increase in Temp. does what to Vapor Pressure? Why? |
Increases Vapor Pressure. Increases number of molecules with sufficient kinetic energy to break the attractive forces that hold them together in the liquid and enable them to enter the gas phase. |
|
|
|
Increasing the surface area does what to vapor pressure? why? |
increases vapor pressure increases the number of molecules on the surface in a population to enter the gas phase. |
|
|
|
Increasing the surface area does what to vapor pressure? why? |
increases vapor pressure increases the number of molecules on the surface in a population to enter the gas phase. |
|
|
|
Increasing the surface area does what to vapor pressure? why? |
increases vapor pressure increases the number of molecules on the surface in a population to enter the gas phase. |
|
|
|
Increasing the surface area does what to vapor pressure? why? |
increases vapor pressure increases the number of molecules on the surface in a population to enter the gas phase. |
|
|
|
Increasing the surface area does what to vapor pressure? why? |
increases vapor pressure increases the number of molecules on the surface in a population to enter the gas phase. |
|
|
|
Increase in intermolecular forces does what to vapor pressure? why? |
decreases vapor pressure increases Kinetic energy needed for molecules to escape surface, and smaller numbers of molecules in the population that have this energy would be there. |
|
|
|
Given Temp and rate constant, what formula would you use to solve for Activation energy? |
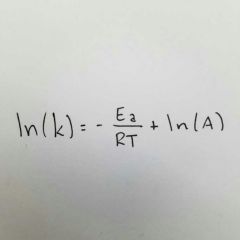
Arrhenius Equation. |
|
|

In an Arrhenius plot graph, which reaction has the greatest activation energy? why? |
D because it has the great slope. m = Ea / R to make m larger, Ea has to increase, R is a constant. |
|
|
|
Define equilibrium in the context of chemistry. |
The Rate forward = rate reverse. Concentrations are not changing. |
|

