![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
62 Cards in this Set
- Front
- Back
|
Scientific Method
|
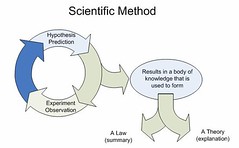
•Observation
•Hypothesis-atentative explanation for observations. Being skeptical •Predicting an outcome that should resultif the hypothesis istrue. •Testing the prediction by an experiment.•Revising the hypothesis.•Testing the new hypothesis and a newpredicted outcome byexperiment•Upgrading the hypothesis to a Theory bymore experiments •Law-asummary of observed behavior |
|
|
Statesof Matter
|
Gaseous Liquid Solid |
|
|
element
|
is a substance that cannot bechemically broken down into simpler substances.
Basic building blocks of matter Composedof single type of atom, like helium |
|
|
compound
|
is a substance composed of two ormore elements in fixed definite proportions.
|
|
|
heterogeneous mixture
|
is one in which the composition variesfrom one region of the mixture to another
|
|
|
homogeneous mixture
|
is one made of multiple substances, butappears to be one substance
|
|
|
Separationof Mixtures
|
Distillation, Filteration
|
|
|
Physical Change
|
Changes that alter only the state orappearance, but not composition
Boiling Water |
|
|
ChemicalChange
|
Changes that alter the compositionof matter are chemical changes.
Rust |
|
|
Potential energy
|
is the energy associated with theposition or composition of an object.
|
|
|
Kinetic energy
|
is the energyassociated with the motion of an object.
|
|
|
Exa- or E |
10^18 |
|
|
Peta- or P |
10^15 |
|
|
Tera- or T |
10^12 |
|
|
Giga- or G |
10^9 |
|
|
Mega- or M |
10^6 |
|
|
Kilo- or k |
10^3 |
|
|
Hecto- or H |
10^2 |
|
|
Deci- or D |
10^-1 |
|
|
Centi or c |
10^-2 |
|
|
Milli- or m |
10^-3 |
|
|
Micro- or U |
10^-6 |
|
|
Nano- or n |
10^-9 |
|
|
Pico- or p |
10^-12 |
|
|
Femto- or f |
10^-15 |
|
|
atto- or a |
10^-18 |
|
|
F to C |
C= (f-32)/1.8 |
|
|
C to K |
K=c+273.15 |
|
|
Density |
D=m/v |
|
|
volume of a sphere
|
V=(4/3)πr^3
|
|
|
volume of a cylinder
|
V=πr^2h
|
|
|
volume of a cube
|
V=a^3
|
|
|
volume of a rectangle
|
V = L * H * W
|
|
|
SignificantFigures Measured
|
values are never 100% precise. In general the moresignificant figures there are in ameasured value, the more precise it is, and the less uncertain it is.
|
|
|
SignificantFiguresExact
|
values have no precision oruncertainty associated with it,they have an unlimited number ofsignificant figures. These numbers are obtained by countingnot using measuring devices. )
|
|
|
SignificantFigures multiplying or dividing
|
•thesame number of significant figures as the measurement with the fewestsignificant figures.
•roundingrules to obtain the correct number of significant figures. |
|
|
SignificantFigures adding or subtracting
|
•thesame number of decimal places as the measurement with the fewest decimalplaces.
•roundingrules to adjust the number of digits in the answer. |
|
|
Accuracy
|
refers to how close the measuredvalue is to the actual value.
|
|
|
Precision
|
refers to how close a series ofmeasurements are to one another or how reproducible they are.
|
|
|
Dimensional Analysis
|
A unitequation is astatement of two equivalent quantities
•A conversionfactor is afractional quantity of a unit equation with the units we are converting fromonthe bottom and the units we are convertingtoonthe top. |
|
|
TheLaw of Conservation of Mass
|
Antoine Lavoisier, This law is consistent with theidea that matter is composed of small, indestructible particles.
|
|
|
TheLaw of Definite Proportions
|
In 1797, a French chemist, JosephProust made observations on the composition of compounds.
All samples of a given compound, regardless of their source or how theywere prepared, have the same proportions of their constituent elements. |
|
|
TheLaw of Multiple Proportions
|
In 1804, John Dalton published his lawof multiple proportions.
When two elements (call them A and B) form two different compounds, themasses of element B that combine with 1 g of element A can be expressed as aratio of small whole numbers. |
|
|
Dalton’s atomic theory
|
1. Each element is composed of tiny,indestructible particles called atoms.
2. All atoms of a given element havethe same mass and other properties that distinguish them fromthe atoms of other elements.
3. Atoms combine in simple,whole-number ratios to form compounds. 4. Atoms of one element cannot changeinto atoms of another element. In achemical reaction, atoms only change theway that they are bound together withother atoms. |
|
|
Millikan’s Oil Drop Experiment
|
American physicist Robert Millikan(1868–1953), performed his now famous oil drop experiment in which he deducedthe charge of a single electron.
By measuring the strength of theelectric field required to halt the free fall of the drops, and by figuring outthe masses of the drops themselves (determined from their radii and density),Millikan calculated the charge of each drop. The measured charge on any drop wasalways a whole-number multiple of –1.96 × 10–19, thefundamental charge of a single electron. |
|
|
Rutherford’s Gold Foil Experiment
|
1. Most of the atom’smass and all of its positive charge arecontained in a small core called a nucleus.
2. Most of the volume of the atom isempty space, throughout which tiny, negatively charged electrons are dispersed. 3. There are as many negativelycharged electrons outside the nucleus as there are positively charged particles (named protons)within the nucleus, so that the atom is electrically neutral. |
|
|
Isotopes
|
Atoms with the same number of protons buta different number of neutrons
|
|
|
Mass Spectrum |
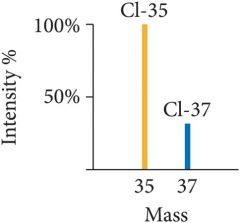
|
|
|
molar mass
|
6.02x 10^23atoms C > 1 molof C atoms > 12.01g of C atoms
|
|
|
convert from g to moles |
divide by molar mass |
|
|
moles to particles |
divide by avagadro’s# |
|
|
particles to moles |
mult by avagadro’s# |
|
|
moles to g |
multiple by molar mass |
|
|
Ionicbonds
|
which occur between metals andnonmetals—involve the transfer of electrons from one atom toanother.
|
|
|
Covalent bonds
|
which occur between two or morenonmetals—involve the sharing of electrons between two atoms.
|
|
|
empirical formula
|
gives the relativenumber of atoms of each element in a compoundnabled
|
|
|
molecular formula
|
gives the actualnumber of atoms of each element in a molecule of a compound-h3
|
|
|
Naming Ionic Compounds
|
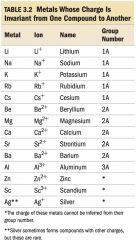
|
|
|
LimitingReactant
|
The reactant that is completelyused up by the reaction, is called the limiting reactant. The excess reactant willremainunreacted.
|
|
|
Theoretical yield
|
always a calculated quantity,calculated by theprinciples of stoichiometry. The mass of products that wecalculated so far was based onstoichiometry there fore, is the geplioahd/extensionLogin.html","title":"LaI
|
|
|
Actual yield
|
is a measured quantity, determinedby experiment.It is usually lower than thetheoretical yield, in actual practicefactors such as impure reactants,incomplete reactions, and sidereactions cause the actual yield tobe lower than theoretical yield.
|
|
|
dilution
|
•water is added.
volume increases. •concentration decreases. |

