![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
26 Cards in this Set
- Front
- Back
|
best study for prognosis
|
cohort
|
|
|
best study for aetiology
|
cohort
|
|
|
best study for intervention
|
RCT
|
|
|
best study for diagnosis
|
diagnostic test evaluation
|
|
|
Levels of Prevention - definition
|
•
Primary prevention - protecting healthy people from developing a disease or experiencing an injury in the first place • Secondary prevention - the goal is to halt or slow the progress of disease (if possible) in its earliest stages • Tertiary prevention – goals include preventing further physical deterioration and maximising quality of life |
|
|
Why we can’t we infer reliable estimates of effect from case reports?
|
1. random error
2. systematic error: - publication bias (more for +ve rather than -ve results) |
|
|
The role of case reports
|
- hypothesis generation
- Awareness raising of rare and unexpected complications of interventions › Convincing when the natural history is uniform and poor and when the intervention is completely and universally effective - The “parachute” effect - Dialysis for symptomatic end-stage kidney disease |
|
|
Why where the HRT for menopause observational studies intially so wrong?
|
- may have been selection bias?
- or they where more health conscious (selection bias) people more likely to get better |
|
|
Role of observational studies
|
› Hypothesis generating for treatment effects
› Essential for evaluating prognosis and therefore who to treat › Often the only evidence we have (simple, feasible) |
|
|
First trial
|
James Lind
1747 6 treatments for scurvy >150yr lag before citrus was used to treat scurvy |
|
|
Formulating a well built question
|
- eg for BP lowering agents we want the outcome to be CVR and not BP lowering because all BP lowering drugs lower BP
PICO |
|
|
A checklist for reporting
(Cochrane risk of bias tool) |
› Were methods for sequence generation reported?
› Was there adequate allocation concealment? › Was there complete outcome data? › Was there intention to treat analysis? › Was there blinding of participants, investigators, outcomes assessors, data analysts? › Was there selective outcome reporting? › Other sources of bias? - Cointerventions - Commercial › Yes, No, Unclear |
|
|
Intention to treat
|
this is when they are given the medication and not checked up to see if they were compliant or not
› Analysis by intention to treat preserves randomization. |
|
|
Bias in treatment assignment in controlled clinical trials
|
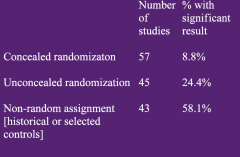
|
|
|
Adequate allocation concealment
|
› Adequate
- central randomisation - numbered/coded bottles/containers - drugs prepared by the pharmacy - serially numbered, opaque, sealed envelopes › Inadequate - alternation, date of birth |
|
|
Should we include?
|
› A patient who ceased medication after only 1% of
medication? › A patient who died before starting randomised treatment? › A patient whose baseline lab results were in error and was ineligible? › A patient who developed cancer shortly after starting treatment? |
|
|
Commercial bias
|
“The sponsor .. has the right to postpone the
publication, for a period not to exceed 18 months.. the sponsor can discontinue the study.. if the aim of the trial has become no longer of interest..a detailed publication policy will be developed..the steering committee agrees to include the modifications requested by the sponsor” |
|
|
What should the comparator be?
|
› Evidence-based standard of care
- Not placebo unless “nothing” is evidence-based standard of care › How can clinicians and policy makers evaluate an intervention against the clinically appropriate comparator when the intervention has only be compared against second-best or placebo? |
|
|
Checklist of thinks to report in a RCT
|

|
|
|
how to read and critique a RCT
|
› Read efficiently – prioritise the abstract, methods, tables+figures
› Comparator: Is it the best available? › Outcome: Is it patient relevant or validated surrogate? › What is the risk of bias? - Randomisation sequence described and valid? - Was there adequate allocation concealment? - Was there blinding x4? - Was there complete outcome data? - Was there intention to treat analysis? - Was there selective outcome reporting? - Was there other sources of bias? - Co-interventions? - Funding? 82 |
|
|
Confidence intervals
|
› Sample size determines precision not validity
- a large trial may be biased › Confidence intervals - if the same study were repeated 100 times then the results of 95 of these 100 studies would lie somewhere within the 95%CI - you can be 95% confident that the true result lies within the 95%CI |
|
|
P-values
e.g P-value = 0.04 |
› p=0.04 means “If the null hypothesis were true (ie no
difference between two groups) then similar data or more extreme to that observed would be obtained 4% of the time by chance alone” › Does not protect against bias |
|
|
P-values from
confidence intervals |
› If the confidence interval includes the “no effect” value
= not statistically significant › If the confidence interval does NOT include the “no effect” value = statistically significant |
|
|
practice question
|
@ th end of the lecture
|
|
|
ARR
|
absolute risk reduction= risk with no Tx - Rsk with tx
|
|
|
NNT
|
1/ARR
|

