![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
21 Cards in this Set
- Front
- Back
|
Ch. 10 Ideal Gas Law |
PV = nRT |
|
|
Ch. 10 P, V, & T |
P1V1 / T1 = P2V2 / T2 |
|
|
Ch. 10 Mole Fraction of Solute |
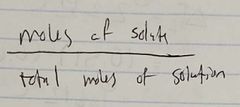
|
|
|
Ch. 10 Mole Fraction of Solvent |
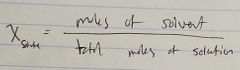
|
|
|
Ch. 10 Relation between mole ratio of Solute and Solvent |
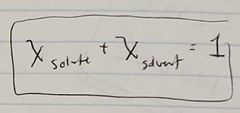
|
|
|
Ch. 10 Molarity |
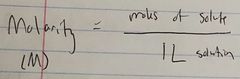
|
|
|
Ch. 10 Molality |
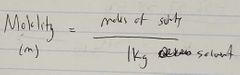
|
|
|
Ch. 10
Mass Percent |

|
|
|
Ch. 10
Roulte's Law |
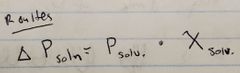
|
|
|
Ch. 11
Endothermic Slopes and Platues |

|
|
|
Ch. 11
Equation for Slope |

|
|
|
Ch. 11
Equation for Platue |

|
|
|
Ch. 11 What are units of "q" in slope/platue stuff |
Q = Kj |
|
|
Ch. 12 Radius of atom in face centered box |
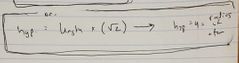
|
|
|
Ch. 12
What are the two different forms of solids |
Amophorphous and Crystaline |
|
|
Ch. 12
What are the 4 types of Crystalline solids |
Molecular Network
Covalent
Ionic
Metallic |
|
|
Ch. 12 Density of face centered atom |
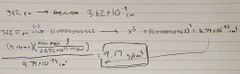
|
|
|
Ch. 13
Osmotic Pressure Equation |
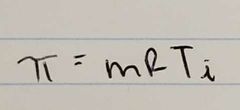
|
|
|
Ch. 13 What are the units of "pi" in Osmotic Pressure Eqiation |
Pi = atm |
|
|
Ch. 13 Freezing Point Depression Equation |
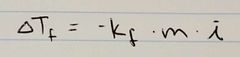
|
|
|
Ch. 13 Boiling Point Elevation Equation |
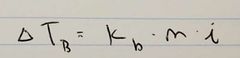
|

