![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
135 Cards in this Set
- Front
- Back
|
What is the diagnostic process
|
1. initial observation
2. recall differential diagnoses 3. rank differential diagnoses 4. collect additional diagnostic information 5. reach a diagnosis |
|
|
What are sources of variation when measuring patient characteristics?
|
1. mechanical
a. machine/equipment b. observer 2. biological a. variation within an animal b. variation between animals |
|
|
Regression to the mean
|
The likelihood of having to elevated (abnormal) test results in a row due to chance is highly unlikely so if a second test shows a more normal result it is likely accurate
|
|
|
diagnostic test
|
anything that will predict the occurrence of a disease with a greater probability than chance alone
|
|
|
screening
|
presumptive identification of unrecognized disease to sort out apparently healthy animals with the disease form those without. screening is done with the objective of early disease detection
|
|
|
case finding
|
when a screening test is applied to a high risk group of animals
|
|
|
What criteria should be considered when evaluating a diagnostic test?
|
- reliability
- accuracy - usefulness - value |
|
|
dichotomous variables
|
variables with 2 possible values (present or absent; alive or dead)
|
|
|
Nominal variables
|
variables that have more than 2 possible values, but not an inherent order (eye color, breed, blood type)
|
|
|
ordinal variables
|
variables that have more than 2 possible values and have an inherent order (pain ratings, grade heart murmur)
|
|
|
accuracy
|
the proportion of all test results, both positive and negative that are correct
(TP + TN)/(TP + TN + FP + FN) |
|
|
sensitivity
|
the ability of a test to accurately classify a group of patients known to have a disease
- ability to correctly identify positive cases TP/ (TP +FN) |
|
|
specificity
|
the ability of a test to accurately classify a group of patients know to be free of disease
- ability to correctly identify negatives TN/(TN + FP) |
|
|
What factors affect whether you choose a test for specificity or sensitivity?
|
- cost of a false negative of false positive
- prevalence of disease - purpose of the test |
|
|
pre-test probability
|
probability that a patient had the disease before the test result was known
- for screening tests it is the prevalence in the population - for diagnostic tests it depends on patient symptoms and other test results |
|
|
post test probability
|
chance of patient having disease after test is run
- postive test= positive predictive value - negative test= 1- negative predictive value |
|
|
predictive value
|
the ability of a test to accurately classify patients whose disease status is unknown
|
|
|
positive predictive value
|
the probability that a patient with a positive test result actually has the disease in quest
TP/(TP + FP) |
|
|
negative predictive value
|
the probability that a patient with a negative test result does not have disease
TN/ (TN+ FN) |
|
|
what is the relationship between predictive value and prevalence?
|
as the prevalence of a disease decreases, the positive predictive value decreases but negative predictive value increases
(if disease is not common the likelihood that a positive result is correct is low while it is more likely that a negative result is accurate) |
|
|
When would you choose a test with high sensitivity?
|
SnNout- sensitivity- negative rules out
- when it is advantageous to rule out a diagnosis in the early stages of a workup to decrease how many animals must be treated - when a false negative could be dangerous, like when screening animals during importation |
|
|
when would you use a test with high specificity?
|
SpPIN- specificity-positive rules in
- when it is advantageous to confirm a diagnosis- confidently determine who should be treated - when a false positive is dangerous- like during a test and cull program |
|
|
natural history of a disease
|
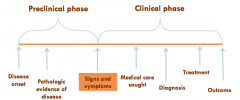
sequence of developments in disease process
- preclinical phase - clinical phase |
|
|
what are possible disease outcomes?
|
- death
- cure - remission (decrease/disappearance of signs and symptoms - recurrence (return of disease) |
|
|
Differentiate between clinical phase of a disease and clinical course of a disease
|
- clinical phase: time frame during which under care
- clinical course: how the disease behaves during the time under care |
|
|
what are the 2 general ways to express prognosis?
|
- # with disease that survived/ # with disease
- # with disease that died/ # with disease |
|
|
case fatality rate
|
method of expressing prognosis
- proportion of patients with disease who die from it |
|
|
one/five year survival
|
method of expressing prognosis
- proportion of patients with disease who are alive one/five years after diagnosis |
|
|
individual years
|
method of expressing prognosis in terms of time
- use # of years in proportion instead of individuals - # years a people with disease survived/ # years every one with disease contributed to study |
|
|
median survival time
|
method of expressing prognosis in terms of time
- length of time that half of the study population survives |
|
|
What is one problem with using person years to express prognosis of disease
|
all person years are assumed to be equal when in fact that may not be.
|
|
|
prognostic factors
|
a factor that may provide information on the likely clinical outcome of each patient
- demographics - disease specific factors - co-morbitidites (other conditions of the patient) |
|
|
cumulative mortality
|
CM= (number of individuals that die during a particular period) / (number of individuals in the population at the beginning of the period)
|
|
|
mortality rate
|
M= (# deaths due to a disease in a population during a particular period) / (total length of time at risk of dying among all individuals in the population)
|
|
|
Death rate
|
total mortality rate for all diseases in a population
|
|
|
What is the relationship between case fatality and case survival?
|
case fatality + case survival = 1
|
|
|
cohort study
|
a group of individuals with the target disorder are followed overtime and the occurrence of the outcome is monitored
|
|
|
censored observation
|
a time measure included for a study subject who does not experience the outcome/event during the observed period
- animal leaves study before experiencing event - animal makes it to the end of study without experiencing event |
|
|
what are some drawbacks of using life tables?
|
- they assume no change in treatment effectiveness or survivorship over time
- they rely upon estimates of time contributed for individuals lost to follow-up and assume they are no different from those still enrolled |
|
|
What is the difference between life tables and the Kaplan Meier Method?
|
Kaplan- Meier Method doesn't use set intervals to estimate probabilities, instead the intervals are determined by the time between occurrence of the observed outcome (e.g. death)
|
|
|
What is an important use of Kaplan Meier curves?
|
they can be used to compare the prognosis of 2 different groups with the same disease
|
|
|
Inception cohort
|
study subjects are all followed from a set point in time in the course of disease
- onset of signs and symptoms - time of diagnosis - beginning of treatment |
|
|
What components determine follow up completeness?
|
- study length- was the follow up period long enough to assure every patient was followed until disease outcome/recovery
- study completeness- were all or a very large proportion of subjects (>/= 80%) followed up for the whole study (not more than 20% lost to follow up) |
|
|
What 3 things must a study reporting prognosis include?
|
- clearly stated zero time (start of tx, diagnosis, appearance of clinical signs)
- long and complete follow-up times - should account for unknown prognostic factors |
|
|
risk factor
|
a factor that has an association with disease or a factor that changes the probability of developing an disease in the future
|
|
|
which increases the pretest probability of a disease more, presence of a risk factor or presence of associated clinical signs?
|
clinical signs
|
|
|
What are some reasons for conflicting results presented in epidemiological studies (e.g. does coffee increase or decrease or have no effect on the risk of cancer in humans)?
|
- limited study populations
- limited variables tested - poor study design - more likely to publish positive associations - random variation |
|
|
association
|
statistical relationship between 2 or more events, characteristics, or other variables
|
|
|
correlation
|
specific type of association in which the relationship between 2 variables is linear
|
|
|
causation
|
a change in one variable is responsible for an observed change in another variable
|
|
|
what are 5 explanations for a causal association observed in an epidemiological study?
|
1. chance (random error): spurious association
2. bias: spurious association 3. effect-cause: real association (heart disease causes obesity) 4. confounding: real association (lack of exercise causes both heart disease and obesity) 5. Cause-effect: real association (obesity causes heart disease) |
|
|
What is chance (in terms of a cause of an association)
|
random errors occur, and are not predictable, but are always present in a study
- can be decreased by increasing sample size |
|
|
What is bias (in terms of a cause of an association)
|
systemic errors that occur when study design, conduct, or analysis mistakenly estimate the relationship of an exposure to an outcome
- unlike chance, this cannot be overcome by increasing sample size |
|
|
confounding factor
|
a factor that is associated with both the suspected cause and the effect being studied
|
|
|
descriptive studies
|
describe population characteristics such as occurrence of disease by time and place
- case report - case series - survey |
|
|
analytical studies
|
examine etiology and causal associations
- observational (observe and analyze natural exposures) - experimental (apply some exposure to a pop) |
|
|
examples of analytical observational studies
|
- cross- sectional
- cohort - case- control - hybrid |
|
|
examples of analytical experimental studies
|
- randomized controlled
- randomized non-controlled |
|
|
In which type of study (analytical or descriptive) can associations be established? why?
|
analytical because in these you are comparing one group to another. In descriptive you are just describing a population so there is no evidence of whether an exposure causes increased risk or not.
|
|
|
case reports
|
descriptive study
- detailed presentation of a single case - usually not representative of normal disease course and not generally very applicable because they focus on unique occurrences |
|
|
case series
|
descriptive study
- description of a collection of cases -- provides evidence/information on the first signs of a disease -- useful in describing the clinical course of disease and natural history of disease -- can provide support for more detailed studies in the future |
|
|
What is the key difference between a cohort study and a case control study?
|
both are observational analytic studies
cohort: identifies exposure and watches what happens case control: identifies people with disease and looks for exposures they had in common |
|
|
cross sectional analytical study
|
Define a set population the identify both exposures and disease in the population at the same time
|
|
|
Types of cohort studies
|
prospective
retrospective |
|
|
prospective cohort study
|
identification and enrollment of cohort is at beginning of time frame under study
|
|
|
retrospective cohort study
|
identification and enrollment of cohort is during or at the end of time frame under study
|
|
|
What is the difference between a retrospective and case control study?
|
retrospective identifies cases based on exposure and case control identifies based on having disease
|
|
|
rank the types of observational studies based on strength of evidence
|
1. prospective cohort (follow up over time)
2. retrospective cohort (follow-up beginning at a later time) 3. case- control ("working backwards") 4. cross- sectional (prevalence and exposure together) |
|
|
what criteria are used to rank strength of evidence in an observational study?
|
1. ability to establish temporal sequence of events
2. risk of bias |
|
|
what are 2 major types of bias?
|
selection
information |
|
|
selection bias
|
absence of comparability between groups being studied
|
|
|
information bias
|
information on exposure, outcome, and covariates of interest is collected differentially between groups
|
|
|
Experimental analytical studies
|
exposure is applied by researcher - can be randomized or nonrandomized- follow-up occurs to determine outcome (typically prospective)
|
|
|
How is incidence used in epidemiological studies?
|
- predict the risk of developing disease
- associate risk factors with disease - predict prognosis - evaluate new therapies |
|
|
relative risk
|
how many times more likely are exposed individuals to become diseased, relative to non-exposed individuals
RR= I_e/I_ne |
|
|
How is relative risk interpreted?
|
RR=1 risk in exposed is equal to risk in non exposed
RR>1 risk is greater in exposed population RR<1 risk is greater in non exposed population |
|
|
attributable risk
|
the incidence of disease that is attributable to exposure
AR= I_e - I_ne |
|
|
population attributable risk
|
predicts the reduction in risk achievable if a risk factor is removed from the population
AR_p= AR x proportion of population exposed |
|
|
Population attributable risk proportion (fraction)
|
measures what proportion of a disease in a population is attributable to a risk factor
AF= AR/I_e |
|
|
Odds
|
ratio of the probability of an event to the probability that the event will not occur
O= P/(1-P) |
|
|
What is the difference between odds and risk
|
denominator
- risk= entire population - odds= those without the outcome |
|
|
odds ratio
|
How many times more likely are diseased individuals to have been exposed, relative to non diseased individuals
OR= O_e/O_ne |
|
|
When would you use an odds ratio
|
instead of relative risk when data on incidence cannot be obtained
- cannot perform a cohort study - disease status is identified first |
|
|
how are odds ratios interpreted?
|
OR=1 likelihood of exposure is equal in diseased and non diseased groups
OR>1 likelihood of exposure is greater in diseased individuals OR<1 likelihood of exposure is greater in non-diseased group |
|
|
What is the fundamental difference in the meaning of relative risk and odds ratio?
|
relative risk= looks at exposure first, how much more likely is disease if you are exposed
odds ratio= looks at disease first, how much more likely was exposure in diseased group |
|
|
What are Koch's postulates?
|
- organism present in every case
- isolate from case and grow in pure culture - organism causes disease when inoculated into a susceptible animal - organism can be recovered from animal and identified |
|
|
what are problems with koch's postulates?
|
- asymptomatic infection negates first rule
- some agents can't be grown in culture - some agents can't be inoculated from isolate - non-infections disease are not subject to the criteria |
|
|
What are hill's criteria?
|
Strong
- temporality - strength of association - consistency - biological gradient Weaker - specificity - plausibility - coherence - experimental evidence - analogy |
|
|
temporality
|
the cause must proceed the effect in time
- the exposure/risk factor must come before the disease |
|
|
strength of association
|
the strong the relationship (RR/OR) between the risk factor and the outcome, the less likely that the relationship is due to something else or by chance
|
|
|
biological gradient
|
increased exposure leads to increased outcome
|
|
|
consistency
|
repeated observation of an association in different populations under different circomstances
|
|
|
specificity of association
|
does the specified exposure lead only to the outcome
|
|
|
biological plausibility
|
does the association make sense in light of existing theories?
|
|
|
Coherence with existing knowledge
|
is the association consistent with available evidence
|
|
|
experimental evidence
|
has a randomized controlled trial been done to support the association
|
|
|
analogy
|
is the association similar to others that have been identified
|
|
|
treat- to -target
|
therapeutic concept that aims to achieve well defined clinically relevant end-targets
- targets are specific quantitative measures with rationale for section based on comprehensive, evidence based, generally accepted values - treatment plans are dynamic and responsive |
|
|
inductive reasoning
|
retrospective analyses of your own clinical experience or pathophysiological reasoning
- bottom up, you make observations and try to find a cause that would fit them every limping dog you have seen has been cured with anti inflammatories therefore anti inflammatories must cure all lameness |
|
|
deductive reasoning
|
analysis of clinical trials, application of prescribed sets of differentials
- top down, Start with something that is known to be true and match your observations to it removing uterus decreases risk and cures pyometra therefore if you spay a dog with a pyometra it will go away |
|
|
faith based decision making
|
recommendation of colleagues, advertisements, or pharmaceutical representative
|
|
|
abductive reasoning
|
starts with an incomplete set of observations and proceeds to the likeliest possible explanation for the group of observations
- observe clinical signs - research possible diagnoses - see which one best matches clinical signs |
|
|
pattern recognition
|
matching diagnosis to "typical" clinical signs of that disease
- more experienced clinicians are more successful |
|
|
Why is randomization useful?
|
- ensures that groups in clinical trials are comparable
- reduces the risk of selection bias - most powerful method of eliminating known and unknown confounding variables |
|
|
What is a method used to ensure balanced groups in a randomized trial?
|
stratified randomization
|
|
|
allocation concealment
|
clinicians are unaware of which treatment the next patient is to receive- different from blinding
|
|
|
How can randomized controlled trials be classified?
|
- by study design
- by outcome of interest - by hypothesis |
|
|
Study design RCT classifications
|
- parallel group
- crossover - cluster - factorial |
|
|
parallel group randomized controlled trial
|
participants are randomly assigned to a group and all participants within a group receive (or don't receive) intervention
most common type |
|
|
crossover randomized controlled trial
|
participates receive (or don't receive) intervention in a random sequence over time
allows the response to different interventions to be observed in individual patients |
|
|
cluster randomized controlled trial
|
pre-existing participate groups are randomly selected to receive intervention
e.g. communities as a whole receive or do not receive and they are compared to other communities |
|
|
factorial randomized controlled trial
|
participants are randomly assigned to a group that receives a particular combination of interventions or noninterventions
very uncommon |
|
|
outcome of interest RCT classifications
|
explanatory
pragmatic |
|
|
explanatory randomized controlled trial
|
test efficacy of an intervention in a research setting, rigorously selected groups and highly controlled
|
|
|
Pragmatic randomized controlled trial
|
test effectiveness of intervention in everyday practice; flexible conditions
- also extra information on likelihood of patients to be compliant with treatment to be gained |
|
|
Classifications of RCT by hypothesis
|
superiority trials
non-inferiority trials equivalence trails |
|
|
types of comparison groups
|
options for "treatments" in a RCT (that aren't the treatment being tested
no intervention observation placebo treatment standard treatment |
|
|
What is the difference between no intervention and observation control groups
|
no intervention- control groups receives no intervention and is not monitored
observation- control group receives no intervention but is closely monitored throughout trial |
|
|
Hawthorne effect
|
the observed effect that is attributable to being included in a scientific study
|
|
|
placebo treatment
|
an inactive substance that is given to mimic the actual treatment being tested
- unethical to use this if there is another proven option for treatment (can't withhold treatment in a life-threatening or serious illness) |
|
|
standard treatment
|
current market leader or the drug that has been averrable the longest
|
|
|
blinding |
individuals involved in the study (patients, owners, clinicians, investigators, etc) are kept unaware of the assigned intervention - single (just patient/owner) - double (patient and clinician) - triple (patient, clinician, and data analyst) |
|
|
what are the benefits of blinding? |
- minimize observation bias - improve patient compliance and retention - reduce co-interventions |
|
|
briefly review protocol for clinical trial |
don't forget to do it |
|
|
What steps should be completed prior to enrolling subjects in a clinical trial |
study design and funding creation of protocol and forms clearance process |
|
|
what is the crucial component in owner/patient consent |
whether the owner understands the risk and benefits of participating in the clinical trial |
|
|
intention to treat analysis |
use information from every subject that was enrolled in study whether they were compliant or not should be done in addition to evaluating based on just who followed through |
|
|
Why is it important to use intention to treat analysis |
if analysis is based only on compliant participant's responses: - the rate of positive response could be skewed by bias - the rate of positive response could be skewed by a confounder associated with compliance |
|
|
P value |
the probability of obtaining the results by observed by chance generally <0.05 is considered significant (results didn't happen by chance) |
|
|
null hypothesis |
the hypothesis that there is no real difference between the 2 groups being compared |
|
|
type 1 error (alpha) |
false positive the risk of concluding that there is a difference in the outcome among groups when there is not usually = 0.05 |
|
|
type 2 error |
false negative concluding that a treatment does not work when it does usually = 0.2 |
|
|
type 1 beta error |
power the probability of being able to identify an effect of treatment, if one exists analogous to the sensitivity of diagnostic tests |
|
|
publication bias |
the greater likelihood that studies with positive results will be published |
|
|
why is publication bias important |
distorts the scientific record influences clinician's decision making hides the "truth" misleads policy makers causes harm to patients |

