![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
49 Cards in this Set
- Front
- Back
|
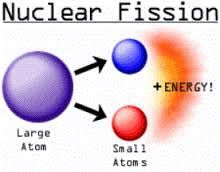
Nuclear Fission |
A heavy nucleus breaks up to form 2 lighter nuclei. |
|
|
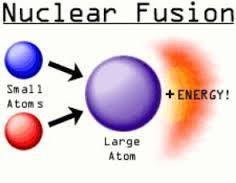
Nuclear Fusion |
Two lighter nuclei combine to form a heavy nucleus |
|
|
Nuclear Fission involves a < BLANK > reaction |
Chain Reaction |
|
|
There is < BLANK > chain reaction involved in Nuclear Fusion. |
No Chain Reaction |
|
|
We have proper mechanisms to control < BLANK > |
Nuclear Fission |
|
|
We do not have proper mechanisms to control < BLANK > |
Nuclear Fusion |
|
|
Disposal of nuclear waste is a < BLANK > with Nuclear Fission |
great environmental problem |
|
|
With Nuclear Fusion disposal of nuclear waste is < BLANK > |
Not involved |
|
|
Raw material for Nuclear Fission is < BLANK > easily available and is costly |
not |
|
|
Raw material for Nuclear Fusion is easily available and comparatively < BLANK > |
Cheap |
|
|
Fission Diagram |
|
|
|
Fusion Diagram |
|
|
|
Nuclear Energy Pros |
|
|
|
Nuclear Energy Cons |
|
|
|
Kinetic Energy |
Energy of Motion |
|
|
All moving objects have |
kinetic energy |
|
|
Kinetici Energy Formula |
2 KE = 1/2 x mass x velocity |
|
|
Kinetic energy can < BLANK > potential energy by increasing the speed of the object or the mass of the object |
increase |
|
|
Potential Energy |
Stored Energy |
|
|
Potential energy is sometimes called < BLANK > |
gravitational potential energy |
|
|
Potential Energy Formula |
PE = mass x height |
|
|
potential energy can be increase by increasing the < BLANK > or < BLANK > |
height or mass |
|
|
Energy |
The ability to do work |
|
|
The ability to cause matter to move or change is < BLANK > |
energy |
|
|
Energy is measured in < BLANK > |
Joules |
|
|
Energy can be calculated using < blank > |
Mathematical Formulas |
|
|
Chemical Energy |
energy stored within the bonds between molecules. example: Natural Gas, Coal, or Gasoline |
|
|
Thermal Energy (heat energy) |
energy of moving molecules example: Fire |
|
|
Mechanical Energy |
energy stored in objects by tension. when the tension is release motion occurs example: a spring |
|
|
Radiant Energy (light energy) |
energy related to the movement of light example: the sun provides radiant energy to warm the earth |
|
|
Electrical Energy |
energy that comes from tiny charged particles called electrons example: lightning |
|
|
Nuclear Energy |
energy created when the nuclei of atoms are split or fused example: nuclear power plants |
|
|
Temperature |
the average kinetic energy of an object |
|
|
SI unit for temperature |
Celsius |
|
|
Heat is < BLANK > energy |
Thermal |
|
|
SI unit for thermal energy |
joules |
|
|
Temperature is not < BLANK > |
Heat |
|
|
Temperature |
average kinetic energy of an object |
|
|
Heat |
thermal energy |
|
|
thermal energy |
total energy |
|
|
same number of particles...higher temperature = |
thermal |
|
|
same temperature...has more particles = |
has more thermal |
|
|
conduction |
transfers heat within a body or between two bodies that are touching. |
|
|
convection |
circular movement that transfer het within air and water |
|
|
radiation |
the transfer of energy by electromagnetic waves through air or space. |
|
|
heat moves one way |
from warmer to cooler until they equal out |
|
|
Thermal Expansion |
as the thermal energy of a substance increases its particles expand or separate |
|
|
Heat Engine |
runs by converting heat energy to work energy |
|
|
Reverse heat engine |
Example: Refridgerator |

