![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
37 Cards in this Set
- Front
- Back
|
How does glucose stimulate release of insulin from beta cells?
|
Glucose enters the beta cell and causes an increase in intracellular ATP. The ATP causes the ATP-dependant K+ channels. Decreased efflux of K+ causes the voltage gated Ca+2 channels to open and Ca+2 enters the cell. The resulting increase in intracellular Ca+2 results in exocytosis of insulin containing granules.
|
|
|
What part of the normal insulin secretion process do insulin secretagogue drugs target?
|
These drugs block the ATP-dependant K+ channel, triggering influx of Ca+2.
|
|
|
What type of glucose receptor is located in all tissues; especially RBC's and the brain?
|
GLUT1
|
|
|
What type of glucose receptor is located in muscle and adipose tissue?
|
GLUT4
|
|
|
What type of glucose receptors are located in the pancreas?
|
GLUT2; this type has the highest transport capability of all the GLUT receptors.
|
|
|
What is the approximate calculation for total daily dose of insulin in diabetics?
|
In units - equal to the person's weight in lbs / 4 OR 0.55 * the person's weight in Kg.
About 1/2 the daily dose covers the background requirements, and the rest covers meal and snack requirements. |
|
|
What conditions would require increased insulin?
|
1. Obesity
2. Adolescence 3. Latter trimesters of pregnancy 4. Type 2 DM |
|
|
What are some signs and syptoms of diabetic ketoacidosis?
|
Nausea, vomiting, abdominal pain, deep slow (Kussmaul) breathing, change in mental status, elevated blood and urinary ketones and glucose, and an arterial blood pH higher than 7.3 and low bicarbonate (under 15 mmol/L)
|
|
|
Treatment for DKA includes what?
|
Aggressive IV hydration and insulin therapy and maintenance of potassium and other electrolyte levels
|
|
|
How does glucagon work?
|
Glucagon binds to a G-protein coupled receptor which leads to an increase in cAMP in the cell. This increase in cAMP facilitates catabolism of stored glycogen and increases gluconeogenesis and ketogenesis.
It works only in the liver - there is no effect on skeletal muscle glycogen because these cells do not have a glucagon receptor. |
|
|
What types of therapy are recommended for the following A1C levels: 6-7, 7-8, over 8.5 and over 10
|
6-7: possible monotherapy
7-8: oral therapy over 8.5: combination over 10: insulin required |
|
|
Metformin is recommended as the first line therapy for obese Type II diabetics; what conditions would change this approach?
|
FYI: Metformin not recommended for Type II diabetics with following complications:
- Renal dysfunction - Congestive heart failure - Untreated alcoholism - If severe illness/sepsis, discontinued until treated - Pregnancy |
|
|
Metformin:
|
Class: Biguanides
MOA: reduces hepatic glucose production through activation of AMP-activated protein kinase. Reduces plasma glucagon levels. Does not depend on functioning beta-cells in the pancreas. Does not induce hypoglycemia. Metabolism: Impairs liver metabolism of lactic acid --> increased risk of lactic acidosis. Clinical Use: First line in type II diabetics. Can also be used in combination therapy. Benefits: May induce weight loss. Decreases A1C by 1-3%. Oral and inexpensive. Drawbacks: Nausea, diarrhea, potentially fatal lactic acidosis. Impairs absorption of B12. |
|
|
What conditions may induce lactic acidosis in a patient on metformin?
|
Kidney disfunction
Alcoholism Dehydration |
|
|
ROSIGLITAZONE and Pioglitazone:
|
Class: Thiazolidinediones
MOA: These drugs target the PPAR-gamma receptor in adipose tissue. They promote glucose uptake and modulate synthesis of lipid hormones and proteins involved in energy regulation. Major effects are an increase in GLUT1 and GLUT4 receptor expression and increase muscle glucose uptake. They also redistrubute fat to decrease insulin resistance. Adverse effects: Weight gain, fluid retention, risk of CHF, increased fractures in postmenopausal females. * Onset of effect is delayed for several months. Benefits: Decreases A1C 1%, lowered triglyceride levels. Contraindications: Pregnancy, liver disease, heart failure |
|
|
Exenatide:
|
Class: Incretin mimic derived from gila monster saliva.
MOA: An analog of glucagon-like-polypeptide 1 (GLP1). It is an agonist at the GLP1 receptor. Potentiates glucose mediated insulin secretion, suppresses postprandial glucagon release, slows gastric emptying and causes loss of appetite. Metabolism: Benefits: Weight loss. Decreases A1C by 1% Side Effects: Nausea, vomiting and diarrhea. Possible fatal necrotizing pancreatitis. Contraindications: Risk of serious renal dysfunction if; concurrent hypertension, Renal impairment, Elderly or severely dehydrated Uses: In conjunction with metformin or sulfonylurea. Is expensive and injectable only. |
|
|
What does GLP-1 do?
|
Incretins like glucagon-like peptide-1 (GLP-1) are released endogenously from the intestine after meals. GLP-1 reduces blood glucose by increasing the production and release of insulin as well as reducing the secretion of glucagon. Endogenous GLP-1 is degraded rapidly in body.
|
|
|
Sitagliptin:
|
An oral GLP-1 therapeutic. Inhibits dipeptidyl peptidase-4 (DPP-4), blocking GLP-1 breakdown to increas GLP-1
|
|
|
GLIMEPERIDE, glipizide and glyburide:
|
Class: Sulfonylureas
MOA: Binds to a sulfonylurea receptor associated with the ATP-sensitive K+ channel on beta-cells. Binding depolarizes the cell, leading to influx of calcium and release of insulin. Also decreases glucacon secretion. Administration: Oral Benefits: Rapid onset, long duration. Lowers A1c by 1-3% Side effects: Weight gain, possible hypoglycemia. Hemolytic anemia if glucose-6-phosphate dehydrogenase (G6PD) deficiency |
|
|
Drugs that act on beta-cells in the pancreas may lose their effectiveness in diabetics over time, why?
|
Because in diabetes there is progressive destruction of the beta-cells, leaving nothing for the drug to work on.
|
|
|
NATEGLINIDE and Repaglinide:
|
Class: Non-sulfur containing insulin secretagogues
MOA: Nateglinide- transient, very rapid but short-acting effect to close K+ channel to increase insulin release (good for postprandial BG surges) Repaglinide- Closes K+ channels, more similar to sulfonylureas Metabolism: via CYP's. Careful when giving CYP inhibitors. Benefits: Lowers A1c by 0.5-1.5% * Used in combo with metformin * Safe to use in patients with renal impairment. |
|
|
Sitagliptin:
|
MOA: Inhibits DPP-4, the enzyme that degrades incretin and other GLP-1 like molecules. This increases circulating levels of GLP-1 and GIP. This increases glucose mediated insulin secretion and decreases glucagon secretion.
Side effects: Nasopharyngitis, upper respiratory infections, headaches, *Pancreatitis and renal impairment* |
|
|
Indications for Insulin use in Type II diabetics:
|
- If oral combinations fail to drop A1c % to target
- Initial A1c > 10 or HHNKS requires rapid treatment - Elderly if resistant to oral hypoglycemics - Type II diabetes progressive Insulin resistance increases with disease duration β cell function decreases with age - Type II oral antidiabetic treatment failure - High post-prandial BG despite oral med’s - Pregnant Type II or gestational diabetes |
|
|
What are the stimuli and inhibitors of insulin release?
|
Stimulus: Glucose, other sugars, leucine, arginine, GLP-1, GIP, CCK.
Inhibitors: Somatostatin, leptin, chronically elevated glucose and FFA's. |
|
|
Binding of insulin to its receptor causes what to happen?
|
Activation of tyrosine kinases on the intracellular part of the receptor --> phosphorylation of insulin receptor substrates (IRS) --> MAPK second messenger activity. This all leads to translocation of GLUT-4 receptors to the cell membrane.
|
|
|
Why are two types of insulin often given to Type I diabetics?
|
Bolus insulin: rapid onset-set, short acting to avoid post-prandial blood glucose (BP) peaks
Basal insulin: Intermediate (NPH) to long-acting insulin to maintain 24 hr glycemic control |
|
|
Rapid acting insulins:
|
- Lispro (15 min onset) ultra short duration (2-4 hrs)..great for post-prandial control, commonly used in insulin pump
- Glulisine (15 min onset) analog of regular insulin approved for injection or use in insulin pump, short duration (2-5hrs) - Aspart (15 min onset); 4-6 hr duration analog of regular insulin; approved for injection or insulin pump |
|
|
Short acting insulin:
|
Regular insulin (30-45 min onset SC) - crystalline
insulin identical to human insulin, short acting (5-7 hrs) Only insulin available for IV administration (Velosulin =approved for pump) 1st line for ketoacidosis or severe hyperglycemia = IV regular Insulin Rapid onset insulin may also be used to reduce ketoacidosis but given subcutaneously |
|
|
Intermediate acting insulin:
|
NPH insulin (Protamine insulin): Decreased solubility/delayed absorption (Onset 1-2 hrs), peaks (4-8)
Type I: Mixed with rapid onset insulin to provide postprandial control –individualized “Basal-Bolus” therapy NPH/regular insulin (70/30 or 50/50) NPH/lispro NPH/Aspart |
|
|
Long acting insulins:
|
Insulin Glargine - slow onset, long-acting (24hr) - maintains continuous level – “PEAKLESS” insulin
Insulin detemir - slow onset, long-acting, similar to glargine with shorter duration (18-22hr); May require 2x’s a day injection. |
|
|
Duration of different insulins:
|
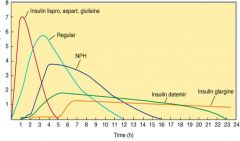
|
|
|
Pramlintide:
|
- Adjunct use with insulin in Type I or Type II diabetics
- Injectable analog of amylin (pancreatic hormone) - Type I diabetics: stabilizes postprandial glucose peaks & valleys - Type II diabetics: suppresses postprandial glucagon secretion & reduces food intake - Slows gastric emptying – may alter absorption of other medications/ nausea adverse effect * Suppresses glucagon release |
|
|
What is hypoglycemic unawareness?
|
An autonomic nervous system neuropathy that prevents diabetics from detecting dangerous hypoglycemia symptoms.
|
|
|
Treatment for hypoglycemia:
|
Conscious: Juice, candy, or high glucose food
Unconscious or vomiting: IV glucose or IM glucagon as soon as possible Glucagon: Potent hyperglycemic appropriate for emergency use to reverse severe insulin-induced hypoglycemia.ONSET is not immediate |
|
|
What is the mechanism of action of glucagon?
|
Glucagon binding to its receptor activates Gs proteins leading to cAMP production --> activation of enzymes that catalyze gluconeogenesis
|
|
|
Name 3 complications that can occur during insulin use:
|
1. Development of IgE antibodies to insulin leading to hypersensitivity reactions.
2. Development of IgG antibodies that neutralize insulin (in obese diabetics) 3. Lipodystrophy: local redistribution of body fat; to avoid, move injections sites around daily. |
|
|
Insulin PK properties:
|
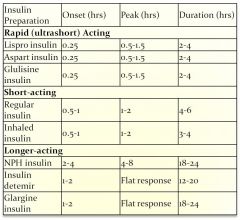
|

