![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
223 Cards in this Set
- Front
- Back
|
What is the embryological derivation of follicular cells of the thyroid gland?
|
Foregut endoderm of the thyroglossal duct from the base of the tongue.
|
|
|
What is the embryological derivation of parafollicular cells (C cells) in the thyroid?
|
Neural crest ectoderm.
|
|
|
What is the embryological derivation of stroma in the thyroid?
|
Mesoderm.
|
|
|
What is the action of calcitonin?
|
Reduces osteoclast resorption, reduces osteoclast numbers, increases renal calcium excretion.
|
|
|
What is the name of the protein that transports iodine across the apical cell membrane of a thyroid follicular cell?
|
Pendrin.
|
|
|
What enzyme within primary lysosomes of a thyroid follicular cell helps cleave thyroglobulin to release T4 and T3?
|
Acid phosphatase.
|
|
|
What is the embryological derivation of the parathyroid glands?
|
Endoderm of the 3rd and 4th pharyngeal pouches.
|
|
|
What are the name of the parenchymal cells in the parathyroid?
|
Chief cells - secrete PTH.
Oxyphils - have no known function. They are non-secretory. |
|
|
What is the most common cause of a midline neck mass?
|
Thyroglossal duct cyst.
The thyroglossal duct is an embryological structure that usually atrophies in week 7 of development. In people that it does not atrophy, cysts can develop within the lumen, and cause a mass. |
|
|
What is the most common place for thyroid ectopia?
|
Base of tongue. This is called a lingual thyroid. Must do imaging before removal, because in 70% of lingual thyroid cases, this is the only thyroid tissue that the patient has.
|
|
|
What is the most common cause of thyroid pain?
|
Painful subacute thyroiditis.
It is a granulomatous inflammatory disorder, that causes transient hyperthyroidism due to destruction of follicles, followed by a period of hypothyroidism that can last for months. Only 5% of patients will have permanent hypothyroidism. May be caused by a virus. |
|
|
In Hashimoto's thyroiditis, which antigens are the autoantibodies directed at, in most cases?
|
90% of cases will have high anti-TPO (thyroid peroxidase).
50% of cases will have high anti-TG (thyroglobulin) |
|
|
What are Hurthle cells?
|
They are abnormal follicular cells in Hashimoto's thyroiditis.
There is an oncocytic change in follicular epithelial cells - they become cells with abundant granular eosinophilic cytoplasm filled with mitochondria. |
|
|
What are the potential long-term complications of Hashimoto's thyroiditis?
|
Non-hodgkin's lymphoma
• 67 X the risk of general population Papillary carcinoma • Minor increase in risk |
|
|
Name the benign and malignant neoplasms of the thyroid gland. What is their cell origin, and prognosis?
|
Benign
- follicular adenoma. Malignant - folliclular carcinoma. Follicular cell origin. Excellent prognosis if total thyroidectomy with radioactive I is given. - papillary carcinoma. Follicular cell origin. Excellent prognosis if lobectomy/total thyroidectomy done with radioactive I, and patient is < 40 years old. Worse if patient is > 40. - medullary carcinoma. Parafollicular C cell origin. Prognosis is good for familial MCT and MEN-2A. Bad for MEN-2B. - anaplastic carcinoma. Likely follicular cell origin. Very bad prognosis. Usually just treat palliatively. |
|
|
Which thyroid carcinomas metastasize via lymphatics? Which ones metastasize via blood?
|
Papillary thyroid carcinoma metastasizes via lymph nodes.
Follicular thyroid carcinomas metastasize via blood (to lung and bones). |
|
|
What is the Wolff-Chaikoff effect?
|
This is when someone is suddenly exposed to high doses of iodide. This inhibits organification of iodide, which in turn decreases thyroid hormone synthesis.
|
|
|
What is the Jod-Basedow effect?
|
When someone that lives in an area with low iodide develops a goiter from long term iodide insufficiency.
If they are exposed to iodide by a medication, or through a change in diet, then they may develop hyperthyroidism. This is called the Jod-Basedow effect. |
|
|
What nuclear imaging is used to diagnose parathyroid gland hyperactivity?
|
Tc99m-MIBI parathyroid scan
|
|
|
How to diagnose the cause of hyperthyroidism, if you already know the TSH is low.
|
It could be Graves' disease, toxic nodular goiter, or thyroiditis.
24-hour thyroid radioiodine uptake will help determine the diagnosis. * if diffuse high intake -> Graves' * if focal high intake -> toxic nodular goiter * if low intake -> thyroiditis causing release of preformed hormone. |
|
|
What effect on blood pressure does high thyroid hormone levels have? low thyroid hormones?
|
TH causes vasodilation -> decreased systemic vascular resisitance. It also increases heart rate and inotropy.
In hyperthyroidism, there is a low diastolic pressure, but high systolic. Wide pulse pressure. In hypothyroidism, there is a high diastolic pressure, and systolic will be not much higher than the diastolic. A narrow pulse pressure, with diastolic hypertension. |
|
|
Name the biological actions of thyroid hormone.
|
Growth and development (brain and bone)
CNS function Cardiovascular hemodynamics (vasodilation, positive inotrope) Female reproductive function Regulation of mitochondrial activity Metabolism in liver * stimulates glycogenolysis, gluconeogenesis, lipolysis, rapid removal of LDL from plasma * inhibits insulin action, immunogenicity. GI - increased gut motility. |
|
|
Multinodular goiter vs. solitary thyroid nodule.
What has a larger risk of being malignant? |
Multinodular goiter runs the exact same risk (~5%) of being malignant as a solitary nodule.
|
|
|
What is the dose of thyroxine given to someone with hypothyroidism due to Hashimoto's thyroiditis?
How often do you monitor and make dose adjustments? |
1.6 mcg/kg/day
Dose adjusted about every 6 weeks, because that's how long it takes for T4 to reach steady states. |
|
|
Within the exocrine pancreas, what are the initial ducts called? What do they drain into?
|
They start as intercalated ducts, which merge into intralobular ducts. Intralobular ducts drain into interlobular ducts between lobules.
Intercalated ducts are low cuboidal. The epithelium changes from low cuboidal to low columnar as the ducts get bigger. |
|
|
What are the four cell types in the pancreatic islets of Langerhans?
|
Alpha - glucagon
Beta - insulin D - somatostatin F - pancreatic polypeptide |
|
|
What is the embryological origin of the posterior pituitary?
|
Neural ectoderm.
|
|
|
What is the embryological origin of the anterior pituitary?
|
Rathke's pouch, an outpouching of oral ectoderm.
|
|
|
What hormones are produced by the intermediate lobe of the pituitary?
|
MSH (melanocyte stimulating hormone)
Beta-endorphin |
|
|
What hormones are produced by the posterior pituitary?
|
Oxytocin
ADH |
|
|
What hormones are produced by the anterior pituitary?
|
GH
LH FSH TSH ACTH Prolactin |
|
|
What is the name of the supportive cells found in the posterior pituitary?
|
Pituicytes. They are similar to glial cells in the CNS. They are supportive cells.
|
|
|
If the pituitary stalk is severed, which hormones will increase?
|
The one hormone that increases is prolactin. This is because prolactin is primarily regulated by inhibition, via dopamine from the hypothalamus. If the stalk is cut, then the inhibition will be lifted, resulting in hyperprolactinemia.
The other hormones will likely decrease. |
|
|
What is the other name for the pineal gland?
What is it derived from? What is its capsule derived from? |
Epiphysis cerebri.
It is a dorsal outgrowth of the brain. Neural ectoderm. The capsule is derived from pia mater. |
|
|
What is produced by pinealocytes?
|
Melatonin, a derivative of serotonin, which has powerful effects on circadian rhythms.
|
|
|
What is the adrenal cortex derived embryologically from?
|
Mesoderm.
|
|
|
What is the adrenal medulla derived embryologically from?
|
Neural crest.
|
|
|
What do the zona reticularis cells contain that makes them stain the way they do?
|
Lipofuscin, a pigment.
|
|

What kind of cells are these?
|
Chromaffin cells in the adrenal medulla.
The one on the left secretes epinephrine, the one on the right secretes norepinephrine. |
|

Which organ is this?
What are the cells on the left? What are the cells on the right? |
Parathyroid gland.
Chief cells on left - secrete PTH Oxyphils on the right - non-secretory function. Unknown function. |
|
|
How does cholesterol from diet get across the enterocyte's brush border?
|
Niemann-Pick C1-like-1 protein.
This is where the drug Ezetimibe works. |
|
|
What apolipoproteins are found on chylomicrons?
|
Apo CII - allows binding and activation of lipoprotein lipase.
Apo B48 Apo E - remnant receptor required for uptake of chylomicron remnants by the hepatocytes. |
|
|
What is the name of the protein that anchors LPL to endothelium?
|
Glycosylphosphatidylinositol-anchored high-density-lipoprotein-binding protein 1
(GPI-HBP1) |
|
|
How do you measure LPL activity in a patient?
|
IV infusion of heparin causes LPL to be released. It must then be phlebotomized to be measured.
|
|
|
What is the apolipoprotein on HDL?
|
Apo A1
|
|
|
What is ABCA-1?
|
It is ATP-binding cassette protein.
Found on almost all tissue cells. It is responsible for binding to Apo A1 on nascent HDL, and transferring free cholesterol as well as phospholipids to HDL. |
|
|
What is LCAT?
|
It is a protein on HDL that esterifies free cholesterol such that it can move into the centre of HDL, making it more spherical.
|
|
|
What is CETP?
|
It is cholesterol ester transfer protein.
It is a protein on HDL that allows HDL to transfer CEs to other lipoproteins such as IDL and LDL. |
|
|
How does HDL deliver CE to the adrenal gland or liver?
|
Via the SRB1 (scavenger receptor B1), which is on adrenal gland cells as well as hepatocytes.
|
|
|
What is the equation used to estimate LDL? What is its limitation?
|
Friedewald equation.
LDL-C = Total-C - (HDL-C + TG/2.2) Does not work if TG is > 4.5 mmol/L, because it assumes that there are no chylomicrons around. |
|
|
What is lipoprotein (a)?
|
It is a subtype of LDL that has Apo(a) (which is structurally similar to plasminogen) linked to Apo B100.
It inhibits the lysis of clots, and is thus thrombogenic. The presence of it is much more atherogenic. |
|
|
What is the best therapy to reduce Lipoprotein(a)?
|
Niacin.
Statins are not that effective - need high dose statins for prolonged periods to see decrease. Aim at lowering overall LDL-C. |
|
|
How long is a:
short chain FA? medium chain FA? long chain FA? very long chain FA? |
Short: 2-5
Medium: 6-12 Long: 13-18 Very long: >18 |
|
|
What is kind of lipid is lecithin?
What is another name for lecithin? |
It is a phospholipid.
Lecithin is also known as phosphatidylcholine. |
|
|
What is the significance of lecithin clinically?
|
It is a marker of fetal lung maturity.
Do amniocentesis and measure lecithin:sphingomyelin ratio. If >=2, then mature. If <1.5, then immature and at risk of neonatal respiratory distress syndrome. |
|
|
What class of lipid is cephalin?
What is another name for cephalin? |
Phospholipid.
Cephalin is also known as phosphatidylethanolamine. Structural membrane PL especially in the CNS. |
|
|
What is the function of phosphatidylserine?
|
It is a membrane structural phospholipid.
|
|
|
What is the function of phosphatidyl glycerol and diphosphatidylglycerol?
What is another name for diphosphatidylglycerol? |
Major component of mitochondrial membranes
Major component of pulmonary surfactant along with diphosphatidylglycerol (cardiolipins) |
|
|
What are the two ether glycerolipids that are important?
|
Plasmalogens, which is component of myelin.
PAF (platelet activating factor) |
|
|
What is the structural backbone of sphingolipids?
|
Ceramine (sphingocine + C22)
|
|
|
Name 4 diseases that result from defects in sphingolipid metabolism.
|
Tay-Sachs disease
Metachromic leukodystrophy Niemann-Pick disease Fabry's disease |
|
|
What is Fabry's disease?
|
A defect in sphingolipid metabolism.
□ X-linked recessive condition □ Due to loss of alpha galactosidase A. □ Causes accumulation of ceramide trihexoside. Clinical manifestations * neuropathic limb pain * stroke * telangiectasia * angiokeratoma in speedo distribution. * renal insufficiency |
|
|
What increases lipolysis?
What decreases lipolysis? |
Stimulants for lipolysis: AAGE
* ACTH * alpha-MSH * GH * Epinephrine Inhibitors of lipolysis: * Insulin Effects of glucagon and cortisol on lipolysis are controversial. |
|
|
What is the mechanism by which GH and epinephrine induce lipolysis?
|
They activate hormone sensitive lipase (HSL), which is an enzyme within adipocytes that cleave TAGs, releasing FFAs and glycerol into blood.
|
|
|
How does FFA get into mitochondrion for beta oxidation?
|

Transported as acyl-CoA through outer mito membrane.
Coupled to carnitine, via CPT-1. Transported through inner mitochondrial membrane as acyl-carnitine. Converted back into fatty acyl-CoA via CPT-II. |
|
|
What are symptoms and sequelae of CPT-I or CPT-II deficiency?
|
Fatality in infancy due to hypoketotic hypoglycemia.
Recurrent myoglobinuria, rhabdomyolysis. Muscle pain, stiffness, tenderness triggered by exercise and fasting. Renal disease may ensue. Very high CK. |
|
|
What are symptoms and sequelae of SCAD, MCAD, or LCAD deficiency?
|
Hypoketotic hypoglycemia.
MCAD is the most common. Will have C8:C10 acyl carnitine ratio increased. |
|
|
Where are VLCFAs metabolized?
|
They occur in peroxisomes.
Adrenoleukodystrophy and Zellweger syndrome are peroxisomal diseases. |
|
|
What is the treatment for myxedema coma?
|
i.v. T4 replacement.
Hydrocortisone iv 100mg q8h for possible coexisting adrenal insufficiency. |
|
|
What age group should have lipids tested?
|
Men > 40
Women > 50 Anyone with certain conditions that predispose them to CVD, regardless of age. |
|
|
What is the international criteria for metabolic syndrome?
|
Central obesity
§ Men >= 94 cm if European ancestry. >= 90 cm for everyone else. § Women >= 80 cm Plus two of the following § HDL-C □ Men < 1.0 □ Women < 1.3 § Triglycerides □ > 1.7 § Hypertension □ BP > 130/85 |
|
|
Which infective agent, and which respiratory condition has been shown by trials to increased atherosclerosis?
|
Chlamydia pneumoniae.
Chronic bronchitis. |
|
|
What laboratory parameters are the best predictors of CAD?
|
Total-C:HDL-C
hsCRP |
|
|
What are the 2009 cholesterol treatment guidelines, based on FRS?
|

|
|
|
What percentage of daily calories should be in the form of fat?
What percentage of daily calories should be in the form of saturated fat? |
25-35%
Keep saturated fats to < 7% of calorie intake. |
|
|
What is the most significant side effect of propylthiouracil and methimazole?
|
Agranulocytosis.
Must discontinue if there is unexplained fever. They both have pregnancy risk factor D, and therefore are not recommended in pregnant women. |
|
|
MOA of anti-thyroid drugs.
|
Methimazole and propylthiouracil inhibit thyroid peroxidase, which is responsible for iodinizing thyroglobulin. PTU has an additional effect of inhibiting conversion of T4->T3.
|
|
|
How does saturated fat affect lipid profile?
|
Saturated fats down-regulate LDL receptors. Therefore, increase LDL-C.
|
|
|
How does fiber, especially soluble fiber, affect lipid profile?
|
Helps inhibit absorption of cholesterol in the GIT.
Increase bile acid excretion Up-regulates LDL receptors |
|
|
What is the main effect of alcohol on lipid profile?
|
High TG, due to increased VLDL formation.
May have small increase in HDL-C in some people. |
|
|
How do phytosterols affect lipid profile?
|
Reduce absorption of cholesterol in GIT
Mainly decrease LDL |
|
|
What are fibrates used for?
Name one fibrate. |
Used to treat dyslipidemia.
Agonist of PPARalpha (peroxisome proliferator activated receptor alpha). * This decreases apoCIII, which is an LPL inhibitor. * Increased LPL activity -> maturation of chylomicrons and VLDL into remnants. PPARa also increases synthesis of apoA-I, A-II, and HDL-C. Overall effect: * large decrease in TG * decreases in TC, LDL-C, ApoB100 * increases in HDL-C Fenofibrate aka Lipidil |
|
|
Name 2 high potency statins, and 1 medium potency statin.
Are they water or lipid soluble? |
High:
* Rosuvastatin (water soluble) * Atorvastatin (lipid soluble) Medium * Simvastatin (lipid soluble) |
|
|
What are the major side effects of statins?
|
Myopathy - elevated CK, rhabdomyolysis.
Hepatic failure - increase ALT and AST. |
|
|
What is the effect of hypothyroidism on lipid profile?
|
Increased LDL-C.
Thyroid hormone is a stimulant for rapid removal of LDL from plasma. |
|
|
What are the major side effects of fibrates?
|
Similar to statins: myopathy, GI, hepatitis, rash.
Unique to fibrates: gout, renal failure. |
|
|
What drug interactions are significant for fibrates?
|
Warfarin
* doses of warfarin will decrease - monitor INR closely. Statins * monitor CK/AST/ALT. Cyclosporin * may precipitate renal failure |
|
|
What does niacin do?
What is its main side effects? |
Inhibits lipolysis.
Lowers triglycerides, increases HDL-C. Main side effect is flushing for about 30 mins after dosing. To prevent, give ASA 30 mins before niacin. Precipitates liver disease, gout, and diabetes mellitus. |
|
|
What is the MOA of Ezetimibe?
What are its side effects? |
Blocks uptake of dietary cholesterol and bile via blockade of NPC1L1 cholesterol transporter on apical brush border of intestine.
Side effects are uncommon. |
|
|
What is the main therapeutic effect of salmon oils?
Name two polyunsaturated fa's contained in salmon oil. |
They inhibit VLDL production, and ultimately result in lowering serum triglycerides.
Eicosapentanoic acid (EPA) Docosahexanoic acid (DHA) |
|
|
What is the mutation in familial hypercholesterolemia?
What is the mode of inheritance? |
Autosomal dominant.
Defective LDL receptor. |
|
|
What is PCSK9 gene mutation?
|
It is an autosomal dominant cause of hypercholesterolemia.
PCSK9 is a protease that degrades LDL receptors. The mutation is an activating mutation, causing increased degradation of LDL receptors, leading to high LDL-C. |
|
|
What is familial combined hyperlipidemia?
|
FCHL is autosomal dominant cause of hyperlipidemia.
Unknown mutation. Higher than normal production of ApoB100. Causes high LDL, high TG. |
|
|
What is familial chylomicronemia?
What is its mode of inheritance? |
Autosomal recessive mutation in:
* LPL * Apo CII * GPI-HBP1 anchoring protein Causes hypertriglyceridemia. Risk of pancreatitis. |
|
|
What disease has a clinical feature of enlarged yellow/orange tonsils?
Pathogenesis of the disease, and pattern of inheritance? |
Tangier Disease.
Autosomal recessive. ABCA1 mutation -> can't transfer cholesterol from macrophage to HDL. Lipid profile: Absence of HDL-C Reduced LDL-C Moderately high TG. |
|
|
What is familial LCAT deficiency?
What is its pattern of inheritance? What other clinical findings can suggest LCAT deficiency? |
Autosomal recessive.
Mutation in LCAT. Causes severely low HDL High phospholipids and unesterified cholesterol. Corneal opacity. Hemolytic anemia. Renal failure. |
|
|
What is familial CETP deficiency?
What is its pattern of inheritance? |
Autosomal co-dominant.
HDL particles become enriched with CEs. High HDL-C High Apo-A1 |
|
|
What disease causes palmar xanthomas?
What is the pattern of inheritance of this disease? Pathogenesis? |
Dysbetalipoproteinemia.
Happens in people who are homozygous for Apo E2. There are three forms of apo E (E2, E3, E4) This disease involves apo E2. Normally, people who are E2 homozygous have lower plasma cholesterol levels. But combined with some other genetic/environmental factors, 1/50 of E2 homozygotes develop this disease where their ApoE has reduced affinity for LDL receptor, and they have increased chylomicron remnants, increased IDLs, and high TG. |
|
|
What is Apo A1 Milano?
|
This is a paradoxical mutation, where the patient has lower HDL, but decreased risk of atherosclerosis.
|
|
|
How does low to moderate intensity activity improve metabolic fitness?
What happens to HDL2/HDL3 levels? |
Activates type I oxidative muscle fibres.
Optimizes use of lipid as primary fuel. Upregulates muscle LPL. Increases insulin sensitivity. Exercise inhibits conversion of HDL2 to HDL3. HDL2 is better at reverse cholesterol transport than HDL3. |
|
|
What major side effects of cholestyramine resins do you need to be aware of?
|
Decrease in INR.
GI - constipation, bloating, cramping. |
|
|
What endocrine disorders can cause corneal opacity?
|
1. Fabry's disease (sphingolipid metabolism defect).
2. LCAT deficiency. |
|
|
What are three external signs of familial hypercholesterolemia?
|
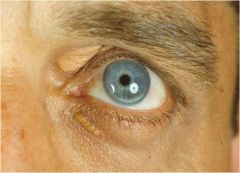
Xanthoma (tendons)
Xanthelasma (around the eyes) Corneal arcus |
|
|
What factors will make the radioactive iodine test inaccurate for diagnosing hyperthyroidism?
|
Note that the scan is done at 4 hours, and 24 hours after ingestion of I-123.
High iodine diet, or iodine containing drug, which competes with I-123 for the thyroid hormone NIS. Iodine deficiency. Extremely hyperactive thyroid, which sucks up all the I and makes TH before the scan is done again at 24 hours. |
|
|
What are some non-hereditary causes of very low HDL-C?
|
Anabolic steroids.
HAART combinations. Combo of fenofibrate and thiazolidinedione. |
|
|
How does cholestyramine resin work?
|
Bind to bile salts, which are -vely charged, and prevent enterohepatic recirculation.
LDL-C decreases because more cholesterol is used to create bile acids, and there is up-regulation of LDL receptors. |
|
|
What is the Wolff-Chaikoff effect?
|
Sudden exposure to excess serum iodide inhibits organification of iodide, thereby diminishing hormone biosynthesis. It is used to treat hyperthyroidism.
|
|
|
Why are glucocorticoids used to treat thyrotoxic storm?
|
Glucocorticoids inhibit T4 to T3 conversion.
|
|
|
What medications are used to treat hyperthyroidism?
|
Propylthiouracil, which blocks throid peroxidase. TPO is an enzyme at the apical membrane of follicular cells, which normally oxidizes iodide, and iodinates thyroglobulin, and also links tyrosines together via ether linkage. It also inhibits peripheral T4 -> T3 conversion.
Carbimazole and methimazole also inhibit TPO, but do not have the same inhibition of T4->T3 conversion. |
|
|
What stimulates insulin release?
What inhibits insulin release? |
Stimulants:
* glucose * glucagon * GIP, GLP-1 * Acetylcholine * amino acids (arginine, lysine) * FFA Inhibitory * Somatostatin * Low glucose * Norepinephrine/epinephrine |
|
|
What stimulates glucagon release?
What inhibits glucagon release? |
Stimulants:
* Low glucose * Norepinephrine, epinephrine * CCK * Amino acid (arginine, alanine) * ACh * GIP Inhibitory: * Somatostatin * GLP-1 * Insulin * High glucose |
|
|
What is the action of islet amyloid polypeptide?
Pancreatic polypeptide? Ghrelin? |
IAPP - inhibits gastric emptying, suppresses appetite. Plays a role in T2DM by forming deposits.
Pancreatic polypeptide - suppresses appetite, inhibits gastric emptying. Ghrelin - stimulates appetite. |
|
|
Which one causes diabetes mellitus - hypo or hyperthyroidism?
|
Hyperthyroidism.
The thyroid hormone causes insulin resistance. |
|
|
What drug(s) are used to treat hyperinsulinism?
|
Diazoxide. Acts on the K+ ATP channel on beta cells, keeping them open, which inhibits insulin release.
Octreotide. Somatostatin analog. |
|
|
Do type 2 diabetics develop ketoacidosis?
|
Usually not, because they have residual insulin around to suppress lipolysis and ketogenesis.
|
|
|
What are the limits for FPG and 2 hour GTT for:
- Normal - Impaired fasting glucose - Diabetes mellitus |
Normal
FPG < 6.1 mmol/L 2 hour GTT < 7.8 mmol/L Impaired fasting glucose FPG between 6.1 and 7.0 mmol/L 2 hour GTT between 7.8 and 11.1 mmol/L DM Classic symptoms and FPG > 7.0 mmol/L Classic symptoms and random plasma glucose > 11.1 mmol/L Classic symptoms and 2 hour GTT test > 11.1 mmol/L |
|
|
What are some acute complications of diabetes mellitus?
|
Diabetic ketoacidosis
Hyperosmolar non-ketotic hyperglycemia. Hypoglycemia Myocardial infarction Infections. Stroke. |
|
|
Why is there a loss of consciousness in hyperosmolar non-ketotic hyperglycemia?
|
It has to do with the hyperosmolar state. The degree of LoC directly correlates with the increase in serum osmolality.
|
|
|
What period of time is HbA1C a measure of average glucose concentration for?
What is the normal range? |
Amount of HbA1C is a measure of average glucose concentration over last 6 weeks.
Normal range is 4-6% |
|
|
ACR is one the lab tests. What is it used for?
|
Urine albumin:creatinine concentration ratio.
* This is to measure the urine albumin excretion ratio. But by dividing by creatinine, it adjusts for volume of urine flow. * Increased albumin excretion is the earliest sign of diabetic nephropathy. |
|
|
Name the two types of progressive glomerulosclerosis seen in diabetes. What are their differences?
|
Diffuse glomerulosclerosis
- diffuse increase in mesangial matrix - basement membrane thickens, but becomes more leaky. - not specific to diabetes. Nodular glomerulosclerosis - rarely causes symptoms - ball-like deposits in mesangial cores. - VERY specific to diabetes. Can make spot diagnosis. |
|
|
What kind of eye disease does diabetes cause?
|
Retinopathy
- background (non-proliferative) - pre-proliferative (trying to grow more blood vessels due to ischemia) - proliferative (neovascularization due to hypoxia) Cataracts Glaucoma Ocular palsies (usually due to CN III infarct) |
|
|
What is diabetic polyradiculopathy?
|

A neurological disease caused by diabetes.
- rapid development of pain, weakness of upper legs. - can lead to muscle atrophy. - usually happens in males with T2DM. - axon loss at nerve root L2-4 |
|
|
These are oral hypoglycemic agents:
Sulfonylurea Meglitinides Biguanides Alpha-glucosidase inhibitors Thiazolidinediones Incretins Which ones don't cause weight gain? |
Alpha-glucosidase inhibitors and incretins are weight neutral.
Biguanides (eg. Metformin) cause weight loss. |
|
|
Sulfonylurea and meglitinides have the same MOA of blocking the K+ ATPase on beta cells. What is the benefit of one over the other?
|
Sulfonylureas are more effective, and longer acting.
Meglitinides can be used in people with renal disease, and has a glucose-stimulated release (so less risk of hypoglycemia) |
|
|
MOA of metformin.
|
Decreases gluconeogenesis
Increases insulin stimulated glucose uptake in muscle and adipose tissue. |
|
|
Name a few of the following insulins that fall under these categories:
Rapid acting (1-2 hours) Regular (4 hours) Intermediate (12 hours) Long acting (24 hours) |
Rapid acting
* Lispro * Glulisine * Aspart Regular * insulin regular Intermediate * NPH Long acting * Glargine * Detemir |
|
|
Draw the intensive dosing regimen for insulin.
|

|
|
|
What happens to serum Na+ and K+ in diabetic ketoacidosis and HHS (aka non-ketotic hyperglycemia)?
|
Usually, hyponatremia and hyperkalemia.
Hyponatremia is caused by dilution due to high serum osmolality. Rarely in HHS, there is an osmotic diuresis that overwhelms the dilutional effect, and the patient becomes hypernatremic. Hyperkalemia ensues from lack of insulin effect on moving K+ into cells. Also, there is solvent drag due to the hyperosmolar state. |
|
|
Why does DKA cause GI pain and vomiting?
|
DKA causes metabolic acidosis and hyperkalemia. Somehow, acidosis and hyperkalemia cause a paralytic ileus.
|
|
|
What is Whipple's triad?
|
Biochemical definition of hypoglycemia:
1. Symptoms of hypoglycemia 2. Lab confirm that glucose < 2.5 mM at time of symptoms. 3. Symptoms abate following rapid administration of glucose. |
|
|
What sort of blood problem does DKA cause?
|
Hypercoagulable state.
|
|
|
What is Addison's disease?
|
Autoimmune adrenal insufficiency.
|
|
|
What is Cushing's disease?
|
ACTH secreting pituitary adenoma.
|
|
|
With an expanding lesion in the pituitary, what is the order in which the loss of pituitary hormones occurs?
|
Go GH
Look LSH For FSH The TSH Adenoma ACTH Please PRL |
|
|
What can cause high prolactin?
What happens in hyperprolactinemia? |
Prolactin secreting adenoma in the anterior pituitary.
Sectioning of the pituitary stalk (prolactin is under inhibitory control by PIF, a dopamine). Hypothyroidism -> high TRH, which stimulates prolactin release. Hyperprolactinemia will: * cause lactation * hypogonadism in males, because prolactin inhibits GnRH release from the hypothalamus. |
|
|
What are the most common hormone secreting tumors in the pituitary? Name them in order of frequency, high to low.
|
Prolactin secreting tumor.
GH secreting tumor. ACTH secreting tumor. |
|
|
What kind of a laboratory test is done to assess general hypopituitarism?
|
Triple bolus test.
Administration of 3 things: 1. Insulin - will lower glucose, causing release of GH, ACTH (cortisol) 2. GnRH - release of LH, FSH 3. TRH - release of TSH, prolactin |
|
|
What are the causes of SIADH (syndrome of inappropriate ADH secretion)?
|
Head trauma, encephalitis, tumors, aneurysms.
Drugs Lung disease Post-operative pain Ectopic ADH (tumors that secrete ADH) |
|
|
What is a serious consequence of chronic hypertriglyceridemia?
|
Pancreatitis.
|
|
|
What is Conn's syndrome?
|
Primary hyperaldosteronism. Hyperplasia of the zona glomerulosa in the adrenal cortex.
|
|
|
What are the main causes of Addison's disease?
|
1. Autoimmune adrenalitis, sometimes associated with APS-II (autoimmune polyglandular syndrome II).
2. Viruses (TB, HIV) 3. Adrenoleukodystrophy |
|
|
What is APS-II (aka Schmidt's syndrome)?
|
Autoimmune polyglandular syndrome II.
These patients will have * Addison's disease * Hashimoto's thyroiditis * Hypoparathyroidism * Diabetes mellitus * Pernicious anemia * Vitiligo * Primary ovarian failure |
|
|
Adrenoleukodystrophy
- pattern of inheritance? - pathogenesis? - diagnosis? |
X-linked inheritance - therefore mostly males
Pathogenesis: deficient in peroxisomal enzyme needed for VLCFA catabolism. Will have accumulation in adrenal cortex, testes, brain white matter, PNS. Diagnosis: VLCFAs (C24, 25, 26s) in plasma, RBCs, WBCs, cultured fibroblasts. MRI shows white matter lesions. |
|
|
What are symptoms of Addisonian crisis?
|
Fever
Dehydration Nausea Vomiting Hypotension Shock Abdominal pain |
|
|
What are causes of secondary adrenal insufficiency?
|
Chronic glucocorticoid therapy.
Pituitary disorders (decreased ACTH) Hypothalamic disorders (decreased CRH) |
|
|
What are two major differences between the clinical features of primary and secondary adrenal insufficiency?
|
In secondary adrenal insufficiency,
* ACTH is suppressed -> no hyperpigmentation. * No symptoms of hypoaldosteronism (eg. hypotension, hyperkalemia), because aldosterone is dependent on RAS axis, which is functional in secondary adrenal insufficiency. |
|
|
What tests is used to determine primary from secondary adrenal insufficiency?
|
Rapid ACTH stimulation test.
* expect cortisol response to be blunted in both primary and secondary insufficiency. In secondary insufficiency, this is because chronic deficiency in ACTH -> adrenal atrophy. * baseline ACTH levels before the test help determine primary from secondary. High ACTH -> primary. Low ACTH -> secondary. To confirm secondary adrenal insufficiency, do an insulin induced hypoglycemia test. Measure ACTH levels before and after. If no rise in ACTH -> confirmed secondary adrenal insufficiency. |
|
|
If a patient with primary or secondary adrenal insufficiency is vomiting (because for some reason, hypocortisolism causes nausea), what is the most important treatment?
|
They must get emergency IV glucocorticoids.
|
|
|
What are causes of primary aldosteronism (aka Conn's syndrome)?
|
Aldosterone producing adenoma (APA).
Idiopathic hyperaldosteronism (IHA). - this is caused by bilateral hyperplasia of the adrenal gland. |
|
|
What are causes of secondary hyperaldosteronism?
|
Renal artery stenosis
Malignant phase of essential hypertension Coarctation of aorta Renin secreting tumor Diuretics that cause Na+ excretion |
|
|
What tests are done to determine if hyperaldosteronism is primary or secondary?
|
1. Aldosterone:Renin ratio. If high -> primary more likely. But need saline infusion suppression to confirm.
2. Saline infusion suppression test, done to confirm primary aldosteronism. - RAS should be suppressed in normal person. - If aldosterone not suppressed -> confirmed primary aldosteronism. |
|
|
If you suspect primary hyperaldosteronism, how do you determine if it is APA (aldosterone producing adenoma) or IHA (idiopathic hyperaldosteronism)?
|
Bilateral adrenal venous sampling for aldosterone.
- APA is usually unilateral. - IHA is usually bilateral. |
|
|
What is the treatment for aldosterone producing adenoma?
How about for idiopathic hyperaldosteronism? |
APA - adrenalectomy
IHA - amiloride, spironolactone, sometimes ACE inhibitors will work. |
|
|
What is used to diagnose congenital adrenal hyperplasia?
|
For 21 hydroxylase deficiency, expect high levels of 17-hydroxyprogesterone.
For 11 hydroxylase deficiency, expect high levels of 11-deoxycortisol. |
|
|
What is the major difference between 21 and 11 hydroxylase deficiency, in terms of clinical features?
|
In 11 hydroxylase deficiency, the patient may present with hypertension, and they are not at risk for Addisonian crisis.
Explanation: 11 hydroxylase results in buildup of 11-deoxycorticosterone, which still has action on the mineralocorticoid receptor. Therefore, it does not result in hypotension, and in fact may present with hypERtension, hypokalemia, and metabolic alkalosis. |
|
|
What is the rate limiting step for biosynthesis of catecholamines?
|
Hydroxylation of tyrosine -> DOPA
Note that this step is inhibited by negative feedback of high levels of E and NE. |
|
|
What is the most sensitive test(s) for diagnosing pheochromocytoma?
|
24 hour urine measurement of:
* metanephrines * catecholamines Note that the level of catecholamines must be 2-3X the upper limit of normal to be specific for pheochromocytoma. |
|
|
What kind of tumors are characteristic of MEN-1 (multiple endocrine neoplasia 1)?
|
Parathyroid
Pancreatic islet cell (insulin, gastrin) Pituitary (prolactin, GH, or ACTH) |
|
|
What kind of tumors are characteristic of MEN-2A (multiple endocrine neoplasia 2A)?
|
Medullary thyroid carcinoma
Pheochromocytoma Hyperparathyroidism |
|
|
What kind of tumors are characteristic of MEN-2B (multiple endocrine neoplasia 2B)?
|
Medullary thyroid carcinoma
Pheochromocytoma Neuromas on lips and tongue Ganglioneuromas of GI |
|
|
What kind of tumors are characteristic of familial MCT (medullary carcinoma of thyroid)?
|
Although it is a subtype of MEN-2, it only results in MCT (medullary carcinoma of the thyroid).
Good prognosis. |
|
|
Which gene mutation is responsible for MEN-2?
Is genetic testing possible? |
Activating mutations of the RET proto-oncogene.
Genetic testing is possible, since the disease is due to a few isolated mutations. Note that inactivating mutations of the RET proto-oncogene results in congenital Hirschsprung's disease. |
|
|
What gene mutation is responsible for MEN-1?
Is genetic testing possible? |
MENIN, a nuclear protein.
There are over 300 mutations, any of which can cause the disease. Therefore, genetic testing is not practical, and annual biochemical screening of high risk individuals is necessary. |
|
|
What is pseudotumor cerebri?
|
It is a rare complication of glucocorticoid withdrawal. Symptoms are raised ICP and papilledema.
|
|
|
What is metyrapone?
|
It inhibits glucocorticoid synthesis. In specific, it inhibits 11-hydroxylation of steroid precursors.
|
|
|
What is ketoconazole?
|
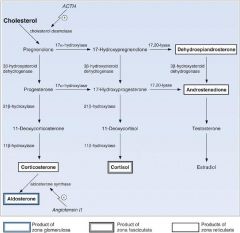
It is an anti-fungal that is also used to inhibit glucocorticoid sythesis. In specific, it inhibits 17-alpha hydroxylase. This prevents steroid hormone precursors to get to the cortisol (as well as the androgen) pathway.
|
|
|
What is mifepristone?
|
Inhibitor of glucocorticoid action.
* progesterone receptor blocker. * glucocorticoid receptor blocker at higher doses. * also used for termination of early pregnancies. |
|
|
A patient has low cortisol. You want to know if it is primary or secondary adrenal insufficiency. You do a rapid ACTH stimulation test. The cortisol does not go up. What does this tell you?
|
You still can't be sure whether this is primary or secondary adrenal insufficiency. In both cases, the cortisol response would be blunted. In primary, because the adrenals are damaged in some way (probably autoimmune, sometimes CAH, adrenoleukodystrophy, or viral). In secondary, the adrenal glands have atrophied.
Thus, you must check the baseline ACTH levels to determine if it is primary or secondary. High ACTH indicates primary adrenal insufficiency. Low ACTH indicates secondary adrenal insufficiency. |
|
|
A patient with Cushing's syndrome is given a CRH stimulation test, and cortisol + ACTH are measured. What do you think the results would be in:
a) adrenal Cushing's (primary)? b) pituitary Cushing's disease? c) ectopic Cushing's? |
a) No response. Production of cortisol by adrenal adenoma is independent of ACTH. Also, the pituitary corticotrophs will have atrophied.
b) Response. The non-tumor, as well as the tumor corticotrophs can respond to CRH stimulation. c) Rare response. In most cases, ectopic ACTH tumors are unresponsive to CRH. In addition, the pituitary corticotrophs will have atrophied. |
|
|
What is the most common cause of Cushing's syndrome?
|
Iatrogenic, secondary to glucocorticoid therapy.
|
|
|
What is the most common non-iatrogenic cause of Cushing's syndrome in women? men?
|
Women: Cushing's disease (aka pituitary ACTH adenoma). They are 8X more likely than men to get Cushing's disease.
Men: ectopic ACTH tumor. |
|
|
How much cortisol normally is bound to proteins in the plasma? Which proteins?
|
90% of cortisol is protein bound. Of the protein bound cortisol, 95% is bound to CBG, 5% by albumin.
|
|
|
How much aldosterone normally is bound to proteins in the plasma? Which proteins?
|
60% of aldosterone is protein bound.
It is bound by both CBG and albumin. Since there is so much more free aldosterone than there is free cortisol, a reduction in the amount of CBG does not have as drastic as an effect on aldosterone as it does on cortisol. |
|
|
How much testosterone is normally bound to proteins in the plasma? Which proteins?
|
98% is protein bound.
60% of testosterone is bound to SHBG. 36% is bound to albumin 1-2% is bound to cortisol binding globulin (CBG) |
|
|
What is the most common symptom associated with pheochromocytoma?
|
Hypertension.
|
|
|
What androgen is secreted in approximately equal quantity to cortisol by the adrenal cortex?
|
DHEA-S
|
|
|
When talking about type 1 diabetes, what is the "honeymoon phase"?
|
When children with T1DM are treated with insulin, their beta cells may start producing insulin again, decreasing the need for exogenous insulin, This phase may last months to several years. Then the last beta cells are killed, and they will remit to hyperglycemic state with increasing exogenous insulin need.
|
|
|
What is the treatment protocol for DKA in a child?
|
- Start an IV and replace with maintenance fluids and fluid deficits using normal saline
- 40 mEq of KCl/L and then - Start an insulin drip at 0.1 units/kg/h 1-2 hours after the start of IV fluids |
|
|
What is the central clock for circadian rhythms?
|
Suprachiasmatic nucleus of the hypothalamus.
|
|
|
Which anterior pituitary hormones is somatostatin capable of inhibiting?
|
GH
TSH ACTH |
|
|
What is the origin of the growth hormone binding protein?
|
The extracellular portion of the GH receptor.
|
|
|
What is the disease caused by a GH secreting tumor?
What medical treatments are available? |
Acromegaly, if the growth plate has closed.
Gigantism, if the growth plate is still open. Bromocriptine (dopamine agonist) Somatostatin analog |
|
|
What factors decrease/increase SHBG in men?
|
These factors increase SHBG in men
• Age, cirrhosis, estrogens, hyperthyroid, HIV These factors decrease SHBG in men • Obesity, glucocorticoids, androgens, hypothyroid, nephrotic syndrome. |
|
|
What is a craniopharyngioma?
|
• They are invasive tumors, but they are histologically benign.
• Usually within the first 2 decades of life. • Develops from remnants of Rathke's pouch. • Slow, relentless growth. • Disturbances in hypothalamic-hypophysial axis, visual system, flow of CSF. |
|
|
What is an important diagnostic test for GH insufficiency?
|
GH stimulation test, using:
arginine, or L-DOPA. Alternatively, can use insulin-induced hypoglycemia, but this is dangerous and must be done under strict supervision. |
|
|
What is the treatment for central diabetes insipidus?
|
Mild cases: Chlorpropamide
Severe cases: ddAVP |
|
|
What is the treatment for nephrogenic diabetes insipidus?
|
Sodium restriction
Diuretics - block sodium resorption. Indomethacin - enhances water resorption, reduction of free water clearance. |
|
|
What is hemochromatosis?
|
It is iron overload, caused by autosomal recessive mutation. Patient cannot regulate iron uptake from intestines.
Iron deposits in liver, pancreas, heart, skin, pituitary. Diagnose: high serum Fe/TIBC, liver biopsy, and C282y genetic test. Treat with phlebotomy. |
|
|
What are the branched chain amino acids?
|
Leucine, isoleucine, valine.
They get used up the quickest in hyperinsulinism, because they are essential a.a. |
|
|
What are clinical features of acromegaly due to GH secreting pituitary adenoma?
|
DM - due to insulin-resistant property of GH.
Arthritis - growth of bones around joints Carpal tunnel syndrome - growth of bone around nerves Hypertension Sweaty skin - sweat gland hyperplasia Large feet, hands, and jaw. Organomegaly. |
|
|
What is the DDx for polyuria and polydipsia?
|
DI (central, nephrogenic, or psychogenic), DM, chronic renal failure
|
|
|
What is the water deficit calculation?
|
0.6 * Pt weight in kg * (measured [Na] / normal [Na] - 1)
Where normal [Na] is 140. |
|
|
What is acanthosis nigricans?
|

It is lesions that are painful, disfiguring, malodorous, or macerated. The lesions are gray-brown to black, rough, and have thickened plaques. They occur in flexural areas, eg. axillary.
Biopsy shows hyperkeratosis, epidermal papillomatosis, and increased melanocytes. Can be seen in obesity, malignancy, and insulin resistance. |
|
|
Does low or high testosterone increase CVD in men?
How about in women? |
Men: low T causes CVD.
Women: high T causes CVD. |
|
|
What is the first line treatment for a pituitary prolactinoma?
|
Cabergoline, a dopamine agonist. Can also use bromocriptine, which is the same thing.
This is because prolactin inhibiting factor, secreted by the hypothalamus, is a dopamine. It is the main inhibitor of prolactin secretion from the pituitary. |
|
|
What is the first line treatment for a pituitary growth hormone secreting adenoma?
|
Surgery (transphenoidal hypophysectomy).
If surgery is unsuccessful, the second line treatment is dopamine agonists, somatostatin analogs, or GH receptor blocker. |
|
|
What are some causes of nephrogenic diabetes insipidus?
|
Hypokalemia, hypercalcemia, sickle cell disease, drugs such as lithium, amyloid, chronic renal failure
|
|
|
How is thyroid hormone transported in blood?
|
> 99% of T3 and T4 are bound to plasma carrier proteins
* TBG binds 75% * Transthyretin binds 10-15% * Albumin binds about 7% * HDL binds 3% |
|
|
What are features of hypercortisolemia?
|
Hypertension
Osteoporosis Growth inhibition Hypogonadism Hypothyroidism Skin, muscle, connective tissue atrophy Diabetes mellitus (insulin resistance) CNS changes (euphoria initially, followed by irritability, depression, etc) Immune system suppression |
|
|
What is responsible for converting cholesterol to pregnenolone, in the synthesis of steroid hormones in the adrenal cortex?
|
ACTH in the ZF and ZR
Angiotensin II in the ZG |
|
|
What increases corticosteroid binding globulin? What decreases CBG?
|
Factors that increase CBG: hyperestrogen state (pregnancy, birth control pills). But free cortisol remains normal due to compensatory over-production.
Factors that decrease CBG: malnutrition, cirrhosis. |
|
|
What stimulates and inhibits aldosterone secretion?
|
Stimulants:
* High K+ * AT II * AT III * ACTH Inhibitors: * ANP/BNP * Low K+ |
|
|
What enzyme stimulates angiotensin II to angiotensin III conversion?
|
Carboxypeptidase
|
|
|
What effect do ANP/BNP have on...
blood vessels? adrenal glands? kidneys? pituitary? |
Blood vessels
□ Vasodilation Adrenal gland □ Inhibit aldosterone release Kidney □ Afferent blood vessels dilate, efferent blood vessels constrict -> increase GFR. □ Inhibit renin release Pituitary □ Inhibit release of ADH and ACTH |
|
|
What are symptoms of Conn's syndrome that are directly due to hypokalemia?
|
Muscle weakness.
Hyperglycemia - for some reason, hypokalemia interferes with insulin release. Nephrogenic diabetes insipidus. |
|
|
What does a pheochromocytoma usually secrete?
|
More norepinephrine than epinephrine.
Sometimes secretes dopamine, or metabolites of catecholamines. |
|
|
What difference does epinephrine and norepinephrine have in terms of action on adrenergic receptors?
|
Same amount of action on all receptors except B2.
E >> NE on Beta-2 adrenergic receptors. |
|
|
What is the normal physiological amount of cortisol that the adrenal glands secrete per day, under non-stressed situations?
|
10 mg/day
|
|
|
What serum or urine tests are ordered to investigate possibility of MEN-2 tumors and carcinoids?
|
Serum calcitonin for MEN-2
Urine 5-hydroxyindoleacetic acid for carcinoids. 5-HIAA is a breakdown product of serotonin. However, this is not a sensitive test. Plasma chromagranin A levels are more sensitive. |
|
|
A patient has papillary thyroid carcinoma, which has metastasized to the cervical lymph nodes. What is the prognosis?
|
The prognosis is still good.
Unique to PTC, spread to cervical lymph nodes does not worsen the prognosis. |
|
|
What is sick euthyroid syndrome?
|
In illness or starvation, the body attempts to lower basal metabolic rate by reducing peripheral conversion of T4 to T3. It increases conversion of T4 to rT3 (inactive). The more ill the patient, the lower the T3.
In severe illness, TSH and T4 can also drop. |
|
|
Why does Addison's disease cause weight loss?
|
Low cortisol results in anorexia.
Dehydration due to lack of aldosterone also contributes. |
|
|
Why do pregnant women get transient hyperthyroidism in their first trimester?
|
hCG has significant homology with TSH. High levels of hCG can interact with the TSH receptor, and stimulate production of T4 and T3.
hCG peaks at 10 weeks gestational age, and levels off after 20 weeks. The hyperthyroidism should resolve spontaneously. |
|
|
What is hypokalemic periodic paralysis?
|
A disorder that happens in Asian males with Graves' disease.
They experience bouts of hypokalemia, with muscle paralysis. When given K+, they have rebound hyperkalemia. |
|
|
What are some functions of sphingolipids?
|
- Intracellular communication
- Antigenic determinant of ABO blood group Also a source of immune vulnerability, as many viruses/bacteria use sphingolipids as their receptors. |
|
|
How does insulin inhibit HSL?
|
It phosphorylates and activated phosphodiesterase type 3B, which decreases cAMP and PKA activity.
cAMP and PKA are how HSL gets activated. |
|
|
Which GLUT is on
- pancreatic B cells? - muscle and hepatocytes? |
- pancreatic B cells: GLUT2
- muscle and hepatocytes: GLUT4 |
|
|
Which is the only statin that is not metabolized by the CYP system?
|
Pravastatin
|
|
|
What is the prevalence of heterozygous familial hypercholesterolemia?
|
1 in 500
|
|
|
What infection can cause diabetes mellitus?
|
Coxsackie virus B4 can attack beta cells and cause DM.
Mumps and Rubella can also cause DM. |
|
|
What drugs can cause impaired glucose uptake?
|
Glucocorticoids
Adrenergic agonists Thiazide diuretics |
|
|
What is the best measure of early diabetic nephropathy?
What drugs are used to control diabetic nephropathy? |
ACR - albumin:creatinine ratio. This adjusts for the urine flow by dividing by creatinine.
Medical management is with ACEi and ARBs. |

